Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 83P
Related questions
Question
Transcribed Image Text:7:39 M
l 65%
on.masteringchemistry.com
:D
<Chapter 15 - Attempt 1
Review | Constants | Periodic Table
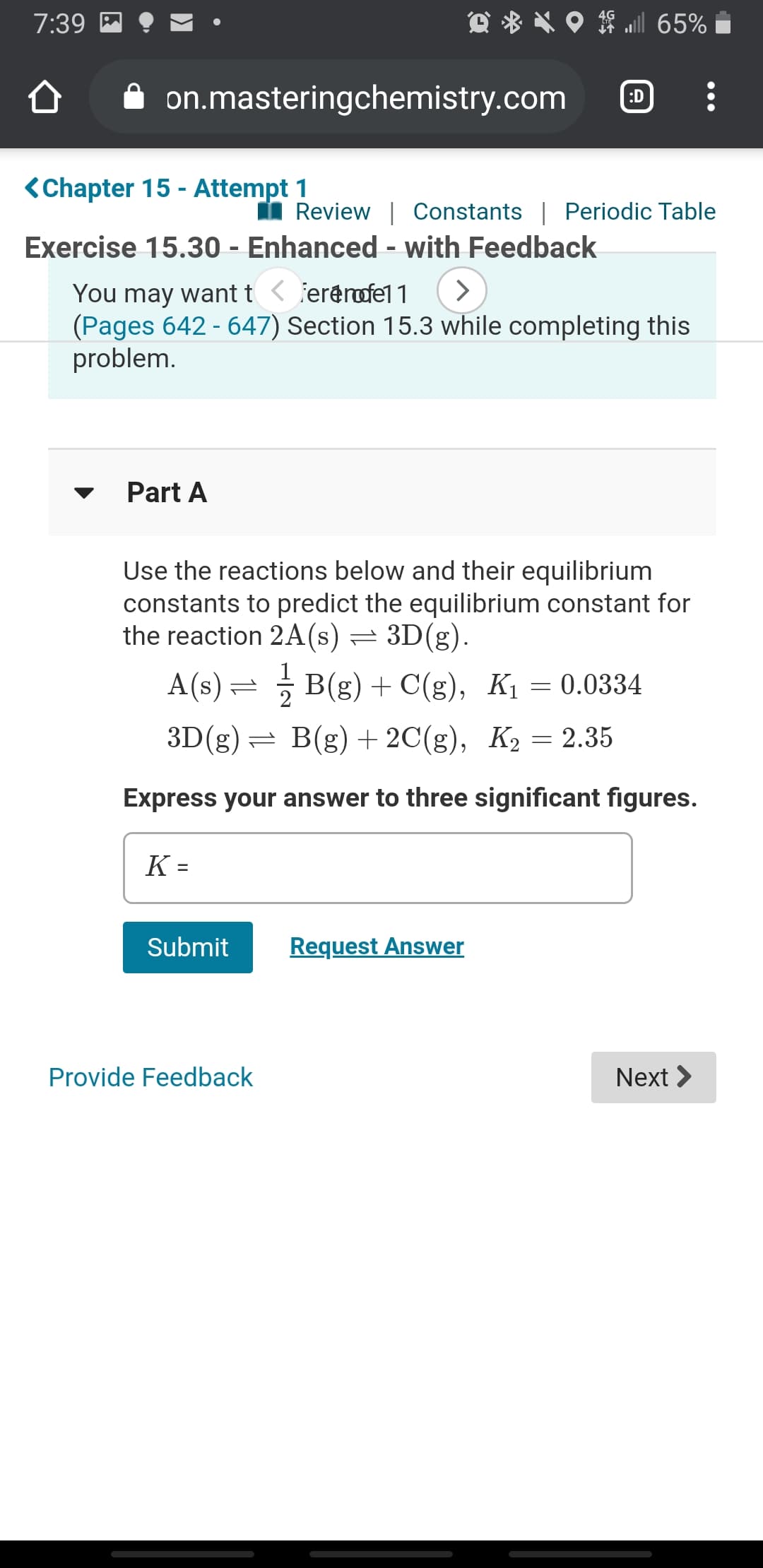
Exercise 15.30 - Enhanced - with Feedback
You may want t < fererofel 1
(Pages 642 - 647) Section 15.3 while completing this
problem.
Part A
Use the reactions below and their equilibrium
constants to predict the equilibrium constant for
the reaction 2A(s) = 3D(g).
A(s) = B(g) + C(g), K1 = 0.0334
3D(g)= B(g) + 2C(g), K2 = 2.35
Express your answer to three significant figures.
K =
Submit
Request Answer
Provide Feedback
Next >
Transcribed Image Text:7:40
l 65%
on.masteringchemistry.com
:D
<Chapter 15 - Attempt 1
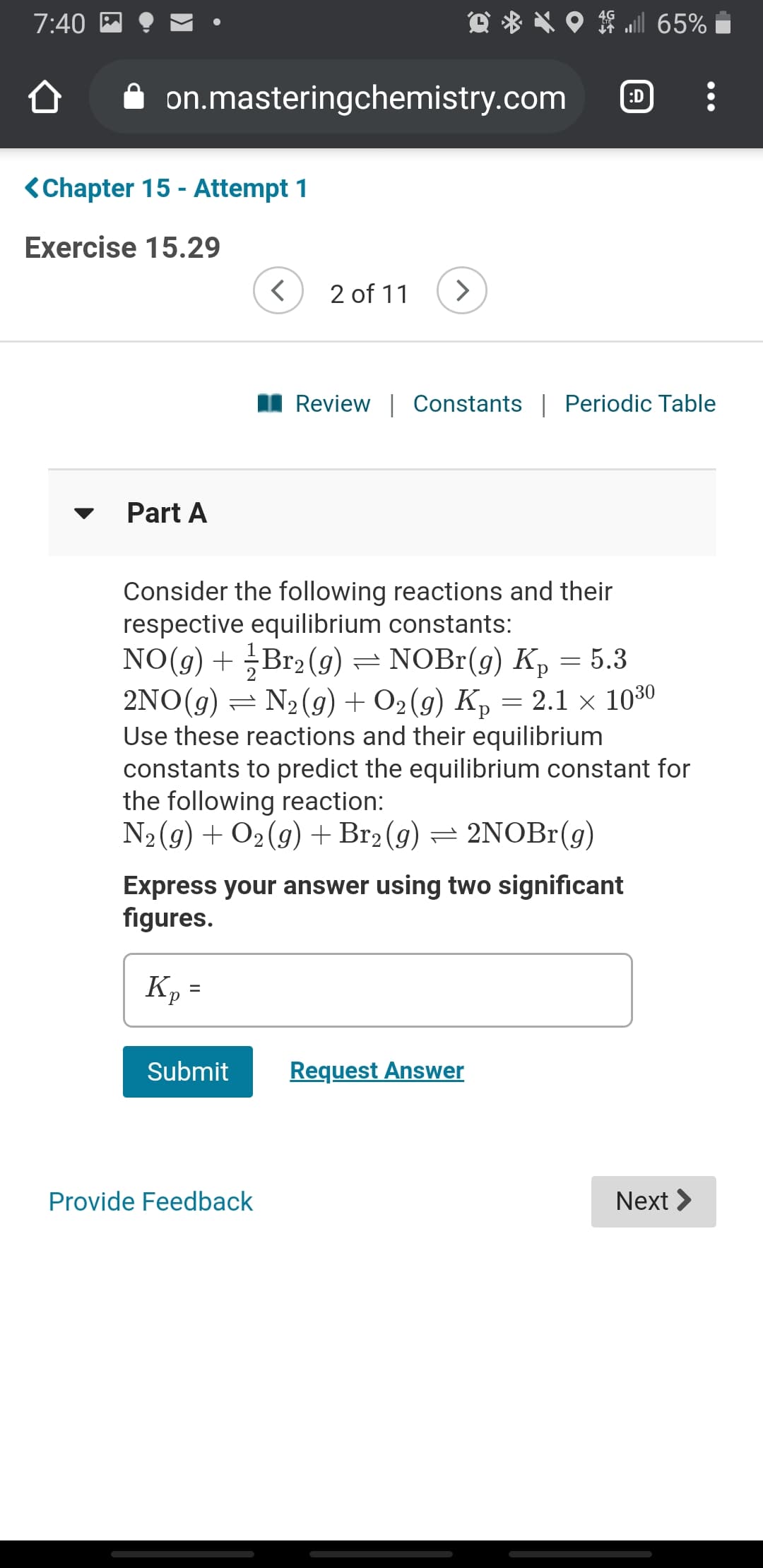
Exercise 15.29
2 of 11
II Review | Constants | Periodic Table
Part A
Consider the following reactions and their
respective equilibrium constants:
NO(g) + Br2 (g) = NOBr(g) Kp = 5.3
2NO(g) = N2(g) + O2 (g) K, = 2.1 × 1030
Use these reactions and their equilibrium
constants to predict the equilibrium constant for
the following reaction:
N2(9) + O2(g) +Br2 (g) = 2NOBr(g)
Express your answer using two significant
figures.
Kp =
Submit
Request Answer
Provide Feedback
Next >
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning