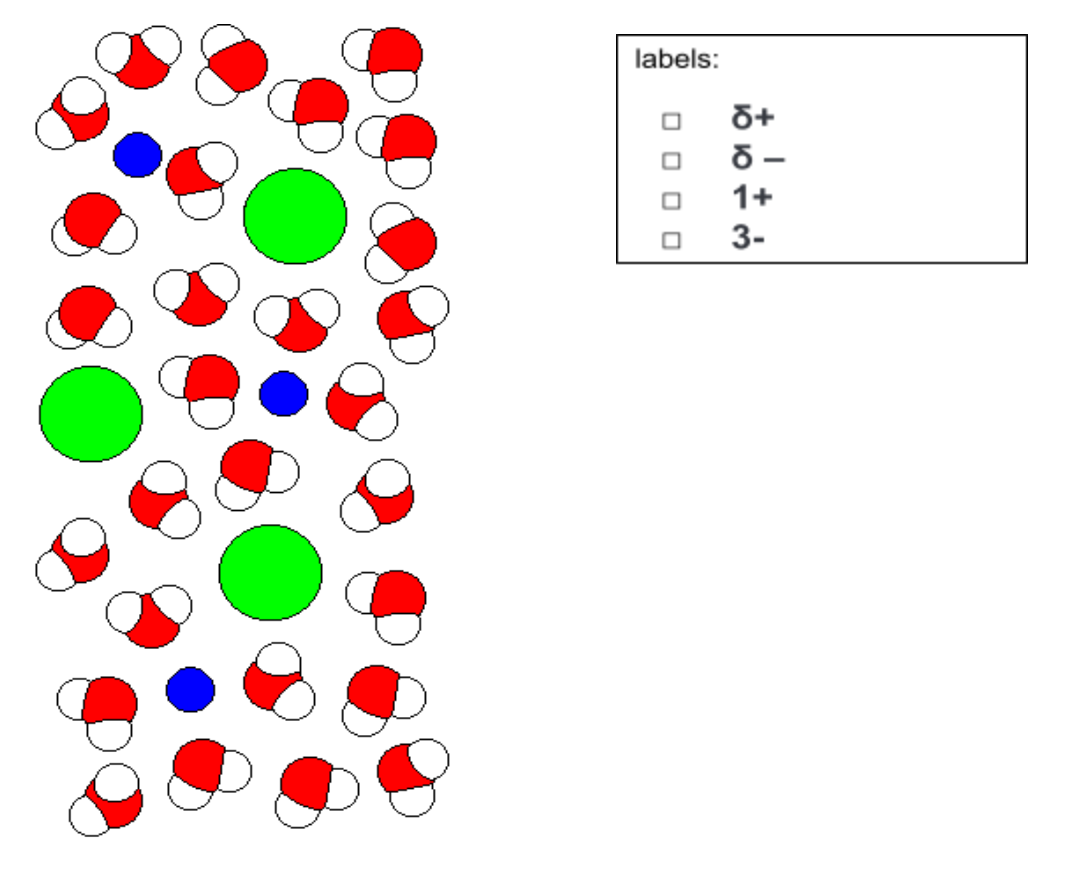
75 mL of 0.300 mol/L sodium phosphate solution is combined with 67.5 mL of 0.350 mol/L calcium bicarbonate. a)Before you begin your reaction, you must accurately produce 1.500 L of your sodium phosphate solution from sodium phosphate trihydrate solid. Write out a procedure to explain all the steps you will take in the lab when making the solution to ensure that your solution concentration is accurate. Please include calculations that show the required mass of solid. Also include the correct names of all equipment used. b)You have a super powerful microscope in your lab! You are able to zoom in on your sodium phosphate solution and take a picture at the molecular level. Label the diagram on the left with the correct choices from the box on the right. You may use arrows or rewrite the symbols in one appropriate place. c)In one sentence, explain what the diagram is showing.
75 mL of 0.300 mol/L sodium phosphate solution is combined with 67.5 mL of 0.350 mol/L calcium bicarbonate.
a)Before you begin your reaction, you must accurately produce 1.500 L of your sodium phosphate solution from sodium phosphate trihydrate solid. Write out a procedure to explain all the steps you will take in the lab when making the solution to ensure that your solution concentration is accurate. Please include calculations that show the required mass of solid. Also include the correct names of all equipment used.
b)You have a super powerful microscope in your lab! You are able to zoom in on your sodium phosphate solution and take a picture at the molecular level. Label the diagram on the left with the correct choices from the box on the right. You may use arrows or rewrite the symbols in one appropriate place.
c)In one sentence, explain what the diagram is showing.
Step by step
Solved in 2 steps with 1 images









