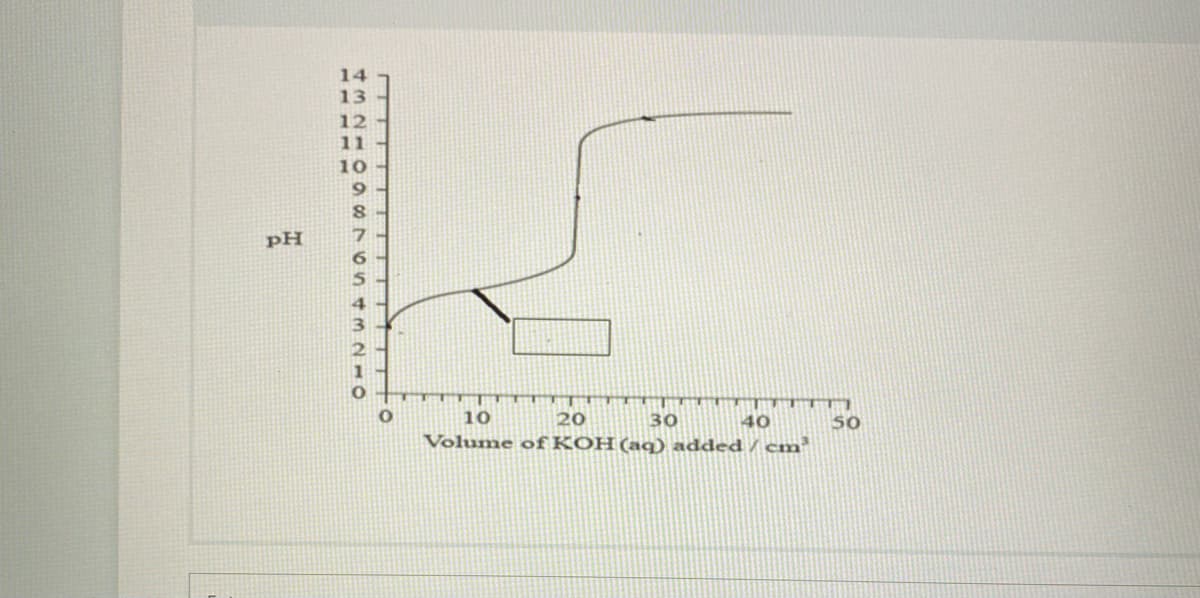
Potentiometric titration curve is given below, which is obtained during the potentiometric titration between strong base KOH (0.2 M) with strong acid HI, label the point in the curve from the following options. If more than one points are present than write as x, y(means separate by using comma) a)The point where pH is because of excess OH - ions. b) The point where pH is only because of HI in water. c)The point where [HI]= [I] in water. d)The point where pH=pka e) The point where all HI is neutralized. f) The point where pH corresponds to solution of [I-] in water. 14 13 12 11 10 9 pH 6.
Potentiometric titration curve is given below, which is obtained during the potentiometric titration between strong base KOH (0.2 M) with strong acid HI, label the point in the curve from the following options. If more than one points are present than write as x, y(means separate by using comma) a)The point where pH is because of excess OH - ions. b) The point where pH is only because of HI in water. c)The point where [HI]= [I] in water. d)The point where pH=pka e) The point where all HI is neutralized. f) The point where pH corresponds to solution of [I-] in water. 14 13 12 11 10 9 pH 6.
Chapter6: Oral Medication Labels And Dosage Calculation
Section: Chapter Questions
Problem 9.5P
Related questions
Question
Transcribed Image Text:14
12
11
10
pH
17
6.
3.
10
20
30
40
Volume of KOH (aq) added / cm
50
![Potentiometric titration curve is given below, which is obtained during the potentiometric
titration
between strong base KOH (0.2 M) with strong acid HI, label the point in the curve from the
following options.
If more than one points are present than write as x, y(means separate by using comma)
a)The point where pH is because of excess OH - ions.
b) The point where pH is only because of HI in water.
c)The point where [HI]= [I] in water.
d)The point where pH=pka
e) The point where all HI is neutralized.
f) The point where pH corresponds to solution of [I- ] in water.
14
13
12
11
10
9
pH
6.
TITIT TIT](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F988f78a0-3428-4c3f-9501-c3cbec48f536%2F354f86e6-9f9e-4e58-8735-40ca61ea36d5%2Fqq74qhr_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Potentiometric titration curve is given below, which is obtained during the potentiometric
titration
between strong base KOH (0.2 M) with strong acid HI, label the point in the curve from the
following options.
If more than one points are present than write as x, y(means separate by using comma)
a)The point where pH is because of excess OH - ions.
b) The point where pH is only because of HI in water.
c)The point where [HI]= [I] in water.
d)The point where pH=pka
e) The point where all HI is neutralized.
f) The point where pH corresponds to solution of [I- ] in water.
14
13
12
11
10
9
pH
6.
TITIT TIT
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

