8. Calculate the concentration in g/100 mL of sugar for each of the solutions prepared. 9. Use the graph of volume ratio vs 1// to determine the volume ratio and then the concentration in g/100 mL of sugar in: a) 100 mL of solution if / is 2.5 cm b) 250 mL of solution if / is 3.5 cm 10. The directions for making a glass of cordial is to dilute the concentrate in the ratio 1:9 (cordial : water). a) How many grams of sugar would there be in a 300 mL glass at this concentration? b) If 1 teaspoon of sugar has a mass of about 4g, the drink will contain how many: i) teaspoons of sugar ii) moles of sugar (sucrose, C₁2H₂2011)?
8. Calculate the concentration in g/100 mL of sugar for each of the solutions prepared. 9. Use the graph of volume ratio vs 1// to determine the volume ratio and then the concentration in g/100 mL of sugar in: a) 100 mL of solution if / is 2.5 cm b) 250 mL of solution if / is 3.5 cm 10. The directions for making a glass of cordial is to dilute the concentrate in the ratio 1:9 (cordial : water). a) How many grams of sugar would there be in a 300 mL glass at this concentration? b) If 1 teaspoon of sugar has a mass of about 4g, the drink will contain how many: i) teaspoons of sugar ii) moles of sugar (sucrose, C₁2H₂2011)?
Chapter8: Sampling, Standardization, And Calibration
Section: Chapter Questions
Problem 8.1QAP
Related questions
Question
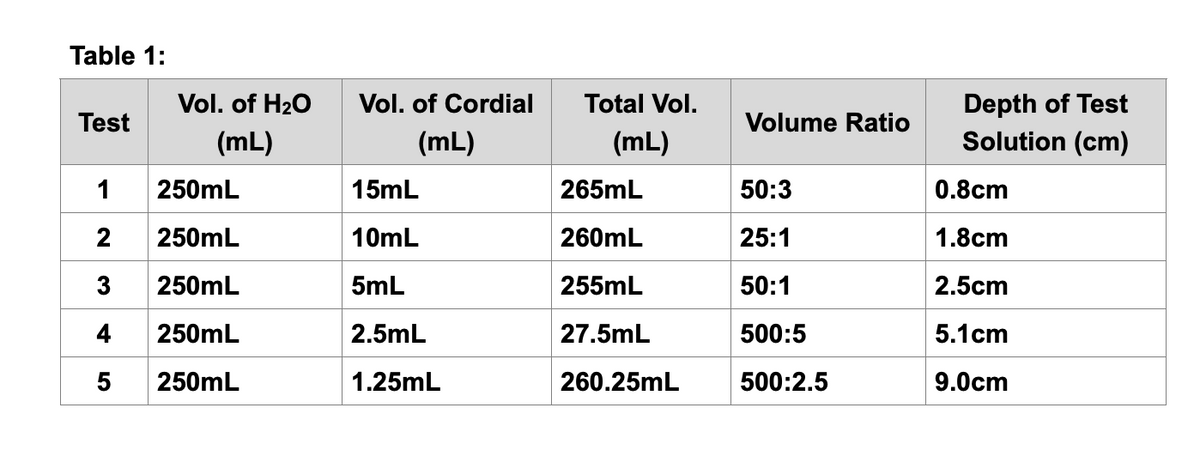
Transcribed Image Text:Table 1:
Test
Vol. of H₂O
(mL)
1
250mL
2
250mL
3 250mL
4
250mL
5 250mL
Vol. of Cordial
(mL)
15mL
10mL
5mL
2.5mL
1.25mL
Total Vol.
(mL)
265mL
260mL
255mL
27.5mL
260.25mL
Volume Ratio
50:3
25:1
50:1
500:5
500:2.5
Depth of Test
Solution (cm)
0.8cm
1.8cm
2.5cm
5.1 cm
9.0cm

Transcribed Image Text:8. Calculate the concentration in g/100 mL of sugar for each of the solutions prepared.
9. Use the graph of volume ratio vs 1// to determine the volume ratio and then the
concentration in g/100 mL of sugar in:
a) 100 mL of solution if / is 2.5 cm
b) 250 mL of solution if / is 3.5 cm
10. The directions for making a glass of cordial is to dilute the concentrate in the ratio 1:9 (cordial :
water).
a) How many grams of sugar would there be in a 300 mL glass at this concentration?
b) If 1 teaspoon of sugar has a mass of about 4g, the drink will contain how many:
i) teaspoons of sugar
ii)
moles of sugar (sucrose, C₁2H22O11)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



