Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter9: Covalent Bonding: Orbitals
Section: Chapter Questions
Problem 44E: Minoxidil (C9H15N15O) is a compound produced by the Pharmacia Upjohn Company that has been approved...
Related questions
Question
KINDLY ANSWER ALL THE FOLLOWING QUESTIONS GIVEN FROM NUMBERS 8 TO 11.
To help me supplement my understanding of the topic. Thank you so much!
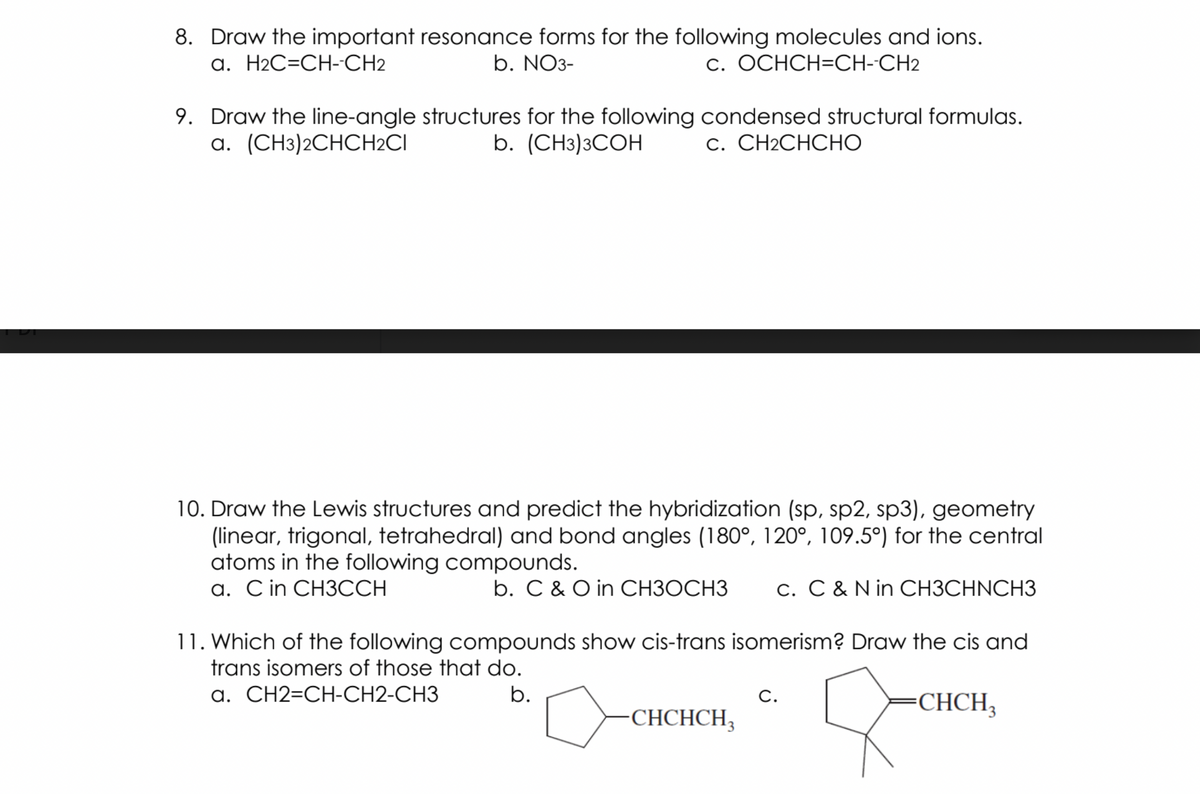
Transcribed Image Text:8. Draw the important resonance forms for the following molecules and ions.
b. NO3-
a. H2C=CH-CH2
c. OCHCH=CH--CH2
9. Draw the line-angle structures for the following condensed structural formulas.
a. (CH3)2CHCH2CI
b. (CHз)3СОН
С. CН2CНСНО
10. Draw the Lewis structures and predict the hybridization (sp, sp2, sp3), geometry
(linear, trigonal, tetrahedral) and bond angles (180°, 120°, 109.5°) for the central
atoms in the following compounds.
a. C in CH3CCH
b. C & O in CH3OCH3
c. C & N in CH3CHNCH3
11. Which of the following compounds show cis-trans isomerism? Draw the cis and
trans isomers of those that do.
a. CH2=CH-CH2-CH3
b.
CHCH,
C.
CHCHCH,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning