8. The following titration data was collected in a lab. Based on the results, determine the concentration of the aqueous sulfuric acid acid. Concentration of NaOH used: 0.50 M Volume of NaOH used: 50.0 mL Indicator: phenolphthalein Endpoint color change: olorless to pale pink Table 1 Volume of sulfuric acid Required Burette reading (mL) Trial 1 Trial 2 Trial 3 Final reading 31.28 39.17 46.70 Initial reading 1.18 9.08 16.62 Volume H:SO4 used (mL)
8. The following titration data was collected in a lab. Based on the results, determine the concentration of the aqueous sulfuric acid acid. Concentration of NaOH used: 0.50 M Volume of NaOH used: 50.0 mL Indicator: phenolphthalein Endpoint color change: olorless to pale pink Table 1 Volume of sulfuric acid Required Burette reading (mL) Trial 1 Trial 2 Trial 3 Final reading 31.28 39.17 46.70 Initial reading 1.18 9.08 16.62 Volume H:SO4 used (mL)
Chapter10: Potentiometry And Redox Titrations
Section: Chapter Questions
Problem 8P
Related questions
Question
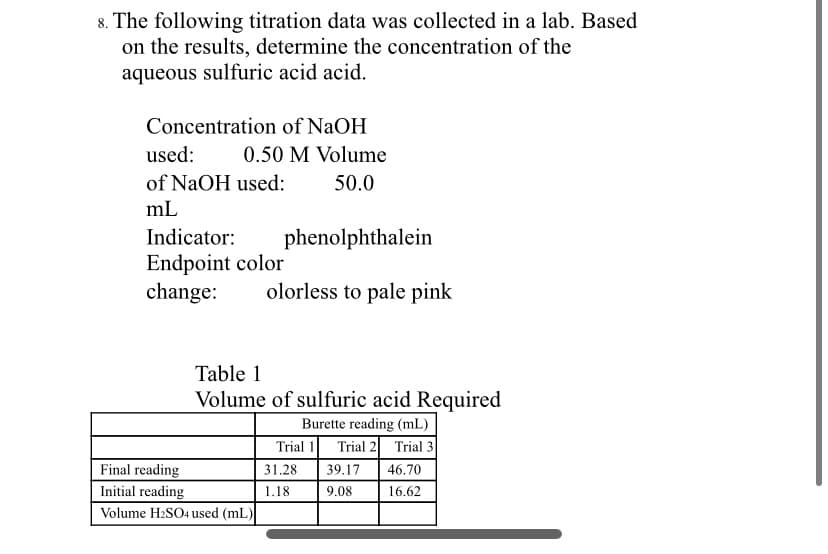
Transcribed Image Text:8. The following titration data was collected in a lab. Based
on the results, determine the concentration of the
aqueous sulfuric acid acid.
Concentration of NaOH
used:
0.50 M Volume
of NaOH used:
50.0
mL
Indicator:
phenolphthalein
Endpoint color
change:
olorless to pale pink
Table 1
Volume of sulfuric acid Required
Burette reading (mL)
Trial 1
Trial 2 Trial 3
Final reading
31.28
39.17
46.70
Initial reading
1.18
9.08
16.62
Volume H2SO4 used (mL)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

