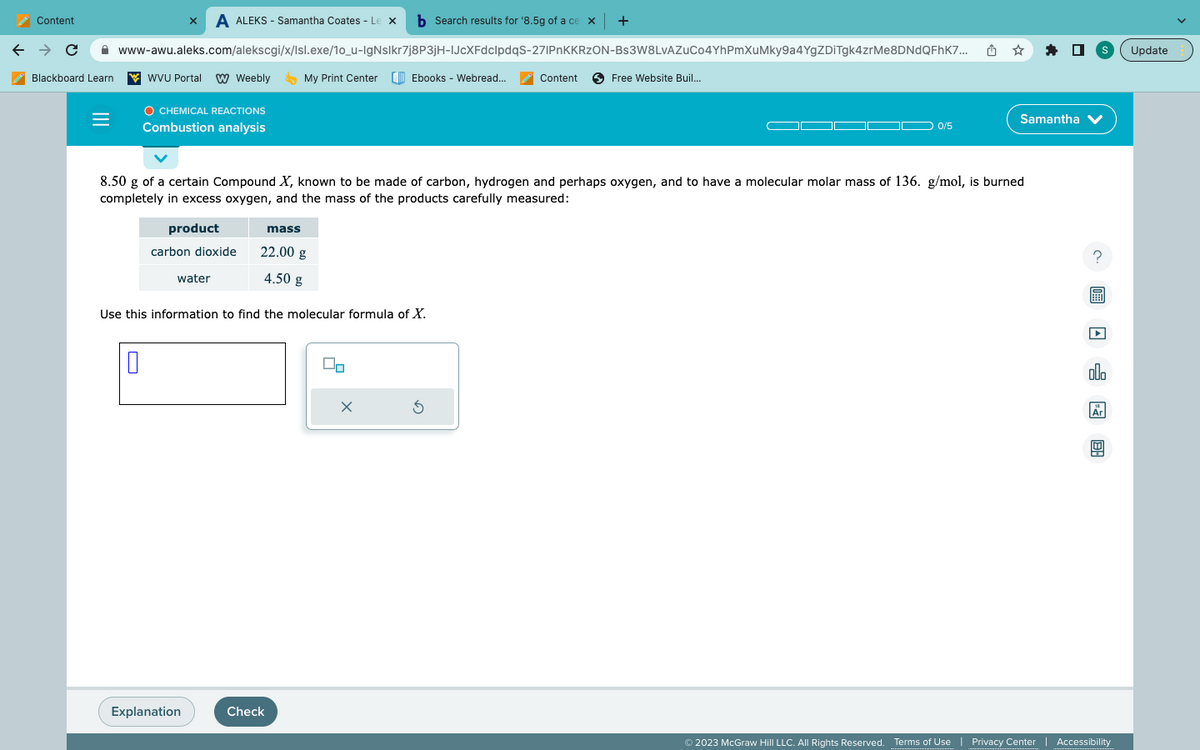
8.50 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 136. g/mol, is burned completely in excess oxygen, and the mass of the products carefully measured:
8.50 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 136. g/mol, is burned completely in excess oxygen, and the mass of the products carefully measured:
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
7.5 gof a certain Compound
X
, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of
70./gmol
, is burned completely in excess oxygen, and the mass of the products carefully measured:
Transcribed Image Text:Content
← → C
Blackboard Learn
=
x A ALEKS-Samantha Coates - Le xb Search results for '8.5g of a ce x +
www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IJcXFdclpdqS-271PnKKRZON-Bs3W8LvAZuCo4YhPmXuMky9a4YgZDiTgk4zrMe8DNdQFhK7...
WVU Portal W Weebly
O CHEMICAL REACTIONS
Combustion analysis
0
water
Explanation
My Print Center [Ebooks - Webread...
mass
Use this information to find the molecular formula of X.
22.00 g
4.50 g
Check
8.50 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 136. g/mol, is burned
completely in excess oxygen, and the mass of the products carefully measured:
product
carbon dioxide
X
Content
5
Free Website Buil....
0/5
ថ
☆
S
Samantha ✔
?
@20:
000
18
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
Update
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The