8.88 A major challenge in implementing the "hydrogen econ- omy" is finding a safe, lightweight, and compact way of stor- ing hydrogen for use as a fuel. The hydrides of light metals are attractive for hydrogen storage because they can store a high weight percentage of hydrogen in a small volume example, NaAlH4 can release 5.6% of its mass as H2 upon decomposing to NaH(s), Al(s), and H2(g). NaAlH4 pos- sesses both covalent bonds, which hold polyatomic anions together, and ionic bonds. (a) Write a balanced equation for the decomposition of NaAlH4. (b) Which element in NAAIH& is the most electronegative? Which one is the least electronegative? (c) Based on electronegativity differences, predict the identity of the polyatomic anion. Draw a Lewis structure for this ion. (d) What is the formal charge on hy- drogen in the polyatomic ion?
8.88 A major challenge in implementing the "hydrogen econ- omy" is finding a safe, lightweight, and compact way of stor- ing hydrogen for use as a fuel. The hydrides of light metals are attractive for hydrogen storage because they can store a high weight percentage of hydrogen in a small volume example, NaAlH4 can release 5.6% of its mass as H2 upon decomposing to NaH(s), Al(s), and H2(g). NaAlH4 pos- sesses both covalent bonds, which hold polyatomic anions together, and ionic bonds. (a) Write a balanced equation for the decomposition of NaAlH4. (b) Which element in NAAIH& is the most electronegative? Which one is the least electronegative? (c) Based on electronegativity differences, predict the identity of the polyatomic anion. Draw a Lewis structure for this ion. (d) What is the formal charge on hy- drogen in the polyatomic ion?
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter20: The Representative Elements
Section: Chapter Questions
Problem 118IP: Although nitrogen trifluoride (NF3) is a thermally stable compound, nitrogen triiodide (Nl3) is...
Related questions
Question
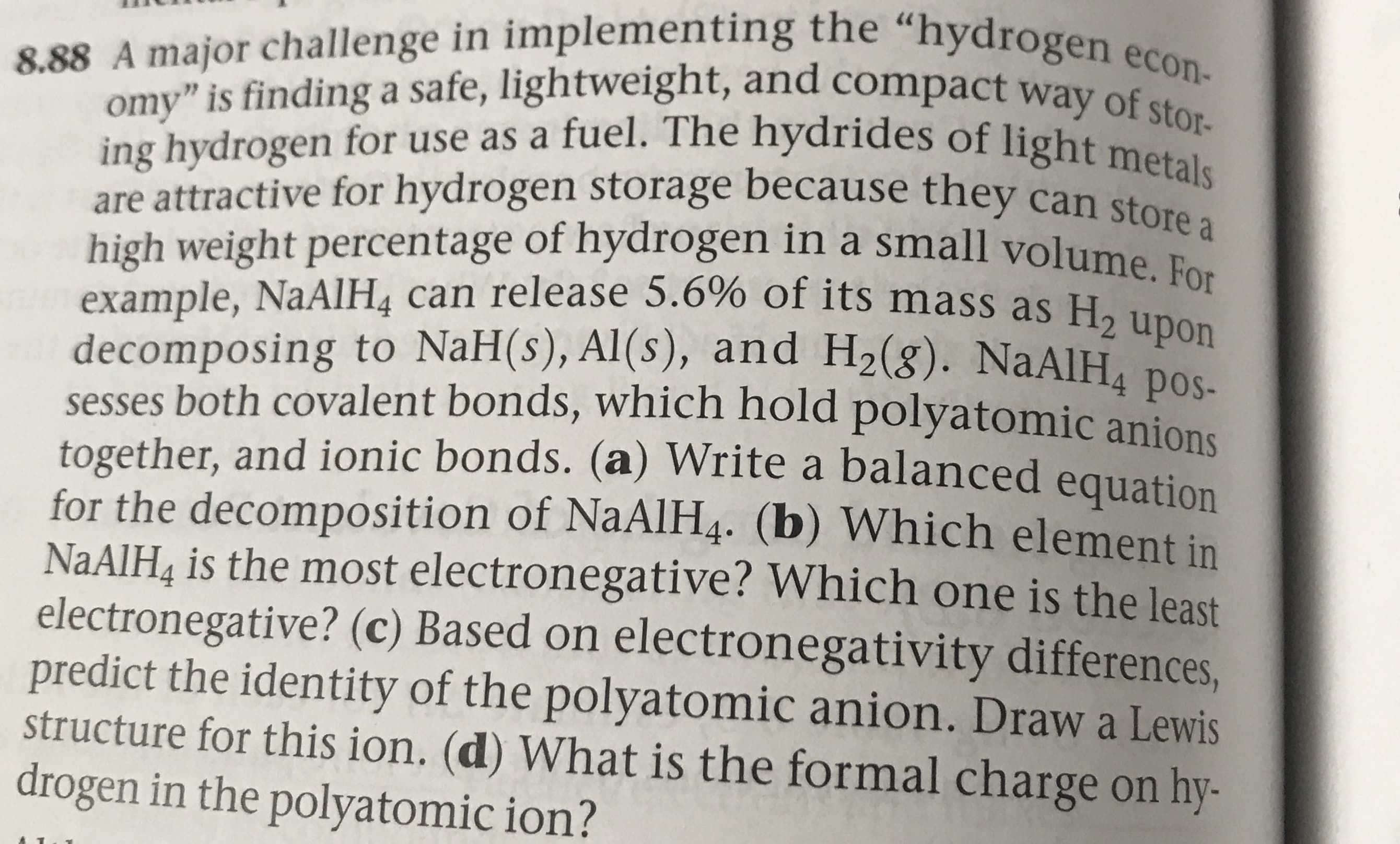
Transcribed Image Text:8.88 A major challenge in implementing the "hydrogen econ-
omy" is finding a safe, lightweight, and compact way of stor-
ing hydrogen for use as a fuel. The hydrides of light metals
are attractive for hydrogen storage because they can store a
high weight percentage of hydrogen in a small volume
example, NaAlH4 can release 5.6% of its mass as H2 upon
decomposing to NaH(s), Al(s), and H2(g). NaAlH4 pos-
sesses both covalent bonds, which hold polyatomic anions
together, and ionic bonds. (a) Write a balanced equation
for the decomposition of NaAlH4. (b) Which element in
NAAIH& is the most electronegative? Which one is the least
electronegative? (c) Based on electronegativity differences,
predict the identity of the polyatomic anion. Draw a Lewis
structure for this ion. (d) What is the formal charge on hy-
drogen in the polyatomic ion?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning