80 но What is the chemical formula for the limiting reactant in the reaction shown? chemical formula: Write the balanced chemical equation for the reaction, using lowest whole-number coefficients. chemical equation: CoCo
80 но What is the chemical formula for the limiting reactant in the reaction shown? chemical formula: Write the balanced chemical equation for the reaction, using lowest whole-number coefficients. chemical equation: CoCo
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter33: Automated Methods Of Analysis
Section: Chapter Questions
Problem 33.6QAP
Related questions
Question
Transcribed Image Text:saplinglearning.com
202-04-12
Chapter Hw
Ivy Tech Community College-..
b Answered. www.Consider the f..
Types Of Diserimination
Learning
ing
Chapter 9 HW
Isab
> Ivy Tech Community College CHEM 111- Spring21- ONJIKO > Activities and Due Dates > Chapter 9 HW
Assignment Score:
28.6%
Resources
Hint
Ch
100%
Question 8 of 14
<>
Correct
100%
Correct
0%
Gave Up
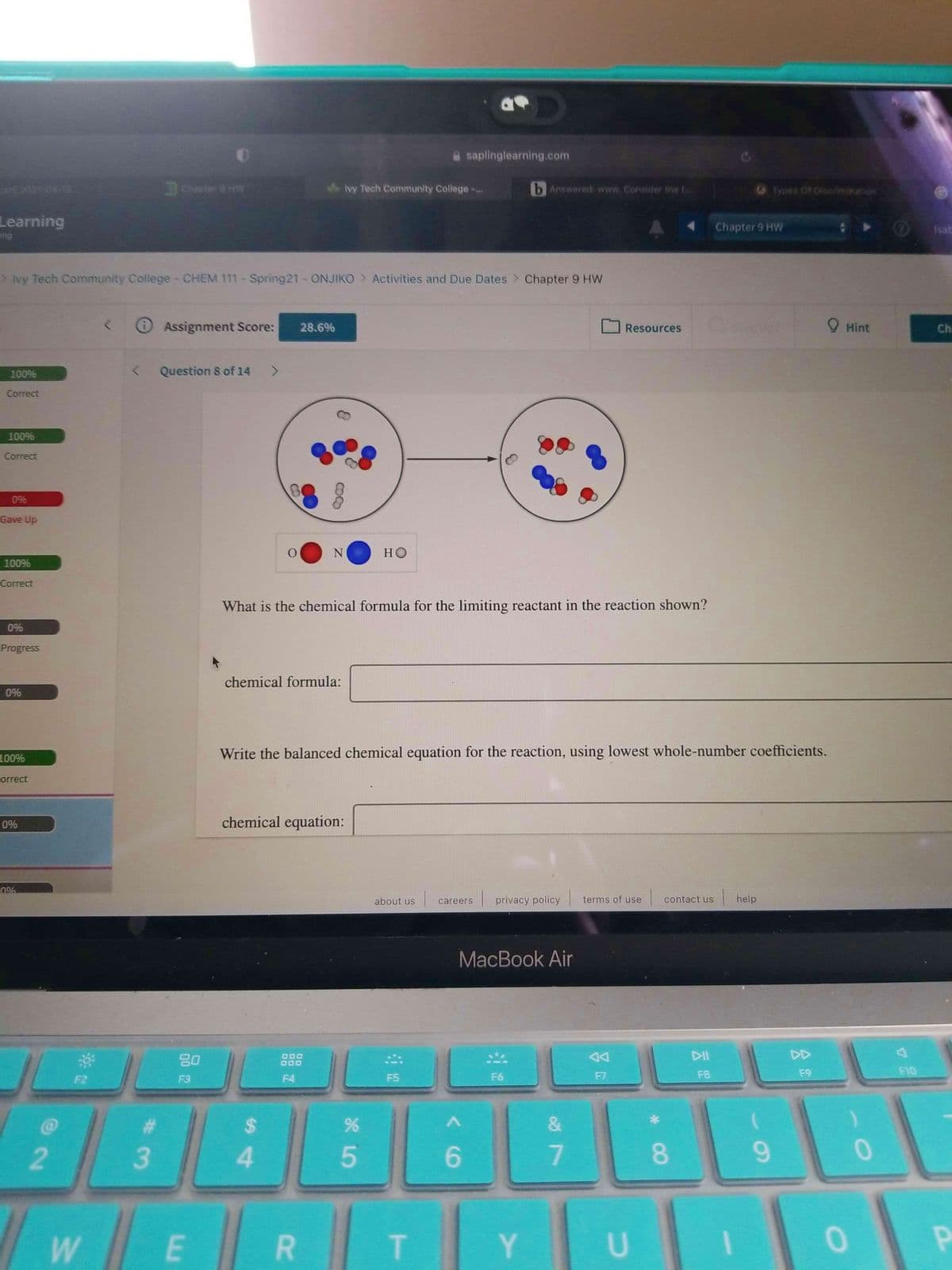
N
но
100%
Correct
What is the chemical formula for the limiting reactant in the reaction shown?
0%
Progress
chemical formula:
0%
100%
Write the balanced chemical equation for the reaction, using lowest whole-number coefficients.
orrect
0%
chemical equation:
|privacy policy
terms of use
|help
about us
careers
contact us
MacBook Air
DII
DD
000
80
F8
F9
F10
F2
F3
F4
F5
F6
F7
%23
%24
&
4.
6.
7.
8.
W
ERT
Y.
3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning