82% Not Secure -openvellum.ecollege.com Course.. Pearson M... Favorites Pearson M... Academic... Engineerin... Maintainin... tate... Career Fai... Help Sign Out Hi, Kali HEM 1210 -Fall 2019 Loza TR 12:45 Course Home
82% Not Secure -openvellum.ecollege.com Course.. Pearson M... Favorites Pearson M... Academic... Engineerin... Maintainin... tate... Career Fai... Help Sign Out Hi, Kali HEM 1210 -Fall 2019 Loza TR 12:45 Course Home
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter33: Automated Methods Of Analysis
Section: Chapter Questions
Problem 33.4QAP
Related questions
Question
100%
Transcribed Image Text:82%
Not Secure -openvellum.ecollege.com
Course..
Pearson M...
Favorites
Pearson M...
Academic...
Engineerin...
Maintainin...
tate...
Career Fai...
Help
Sign Out
Hi, Kali
HEM 1210 -Fall 2019 Loza TR 12:45
Course Home
<Homework 3.4-3.6 for Mon Sep 9
1 of 8
Problem 3.32 Enhanced with Feedback
Part A
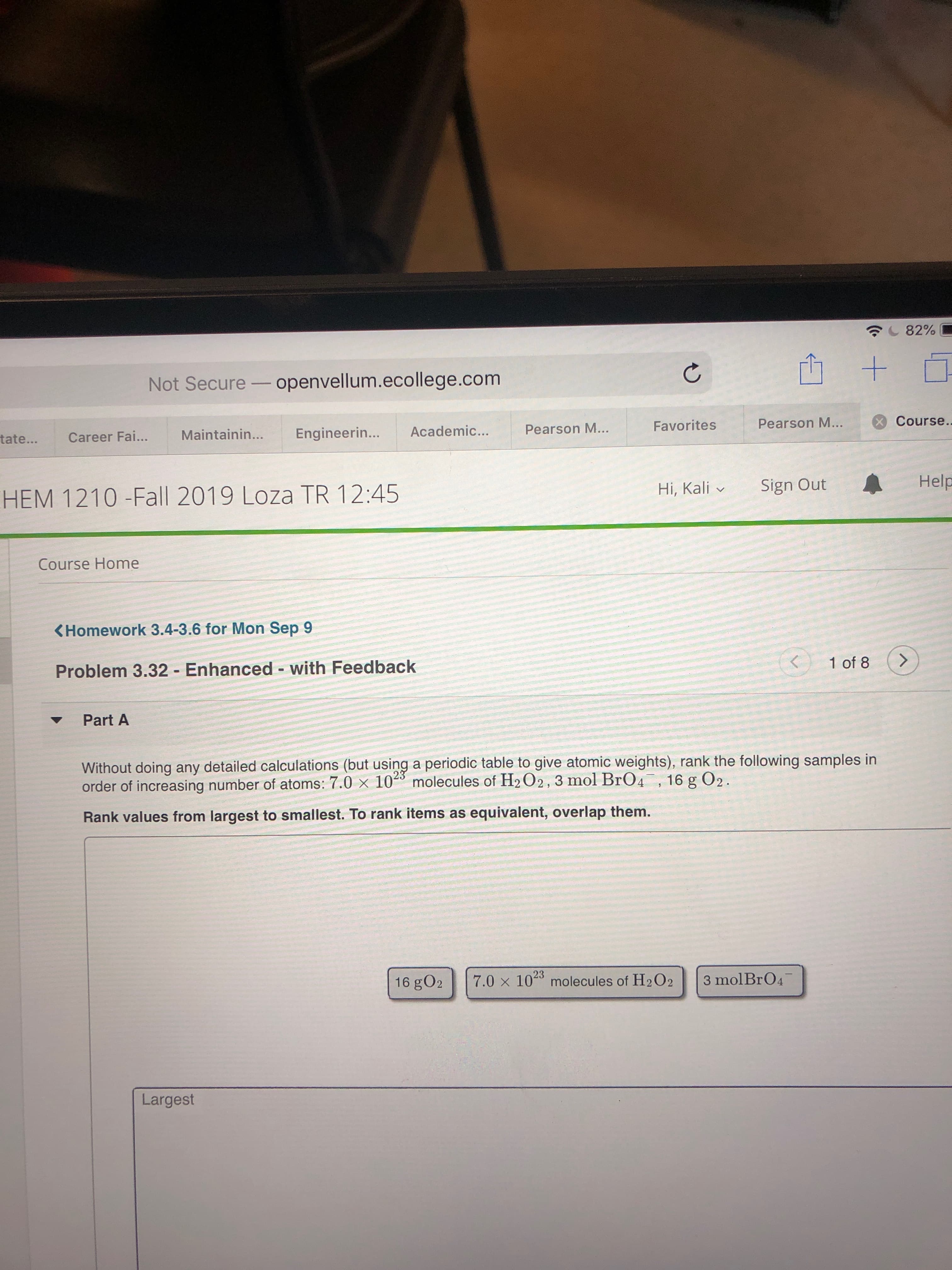
Without doing any detailed calculations (but using a periodic table to give atomic weights), rank the following samples in
order of increasing number of atoms: 7.0 x 10 molecules of H2 O2, 3 mol BrO4, 16 g O2.
23
Rank values from largest to smallest. To rank items as equivalent, overlap them.
3 mol BrO4
7.0 x 102 molecules of H2O2
16 gO2
Largest
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning