(9) At 300 K, the second virial coefficient (B) for N2 is –4.2 cm3 • mol-1, while for CH4 it is – 15 cm³ • mol-1. Which gas behaves less like an ideal gas at this temperature? (MAKE SURE TO EXPLAIN YOUR REASONING).
(9) At 300 K, the second virial coefficient (B) for N2 is –4.2 cm3 • mol-1, while for CH4 it is – 15 cm³ • mol-1. Which gas behaves less like an ideal gas at this temperature? (MAKE SURE TO EXPLAIN YOUR REASONING).
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter1: Gases And The Zeroth Law Of Thermodynamics
Section: Chapter Questions
Problem 1.11E: What is the value of FP for a sample of gas whose temperature is -33.0 C and volume is 0.0250 L?...
Related questions
Question
100%
please typing in clear font or computer
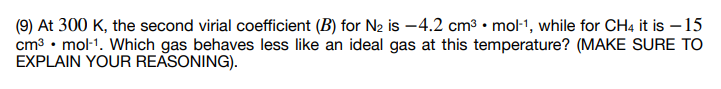
Transcribed Image Text:(9) At 300 K, the second virial coefficient (B) for N2 is –4.2 cm3 • mol-1, while for CH4 it is – 15
cm3 • mol-1. Which gas behaves less like an ideal gas at this temperature? (MAKE SURE TO
EXPLAIN YOUR REASONING).
Expert Solution
Step 1
virial equation of state for 1 mol of any gas is
PV=RT +
Where V= v/n
Where P = pressure of the gas , T= temperature in K ,R = universal gas constant
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning