Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter6: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 142IP: Tris(pentatluorophenyl)borane, commonly known by its aeronym BARF, is frequently used to initiate...
Related questions
Question

Transcribed Image Text:12) A sample of pure sodium carbonate, NazCO3, weighing 0.3542 g is dissolved in water and
titrated with a solution of hydrochloric acid. A volume of 30.23 ml is required to reach the
methyl orange end point, the reaction being
NazCO3 +2HCI →2NACI + H20+ CO2
Calculate the molarity the normality of the acid
(a) Using moles at the equivalence point
(b) Using equivalents at the equivalence point
.ilibriunm
atrations
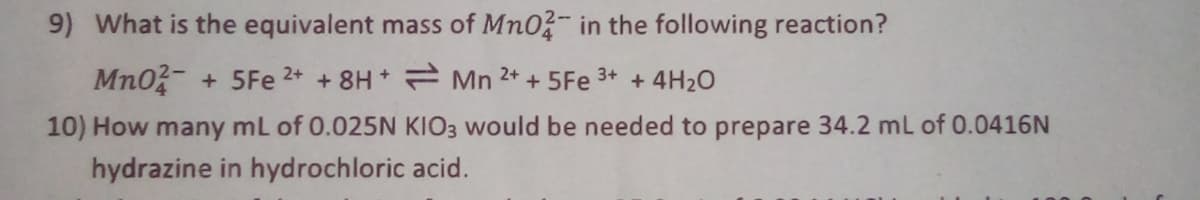
Transcribed Image Text:9) What is the equivalent mass of Mn0?- in the following reaction?
Mn0- + 5Fe 2+ + 8H Mn 2+ + 5Fe 3+ + 4H2O
10) How many mL of 0.025N KIO3 would be needed to prepare 34.2 mL of 0.0416N
hydrazine in hydrochloric acid.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning