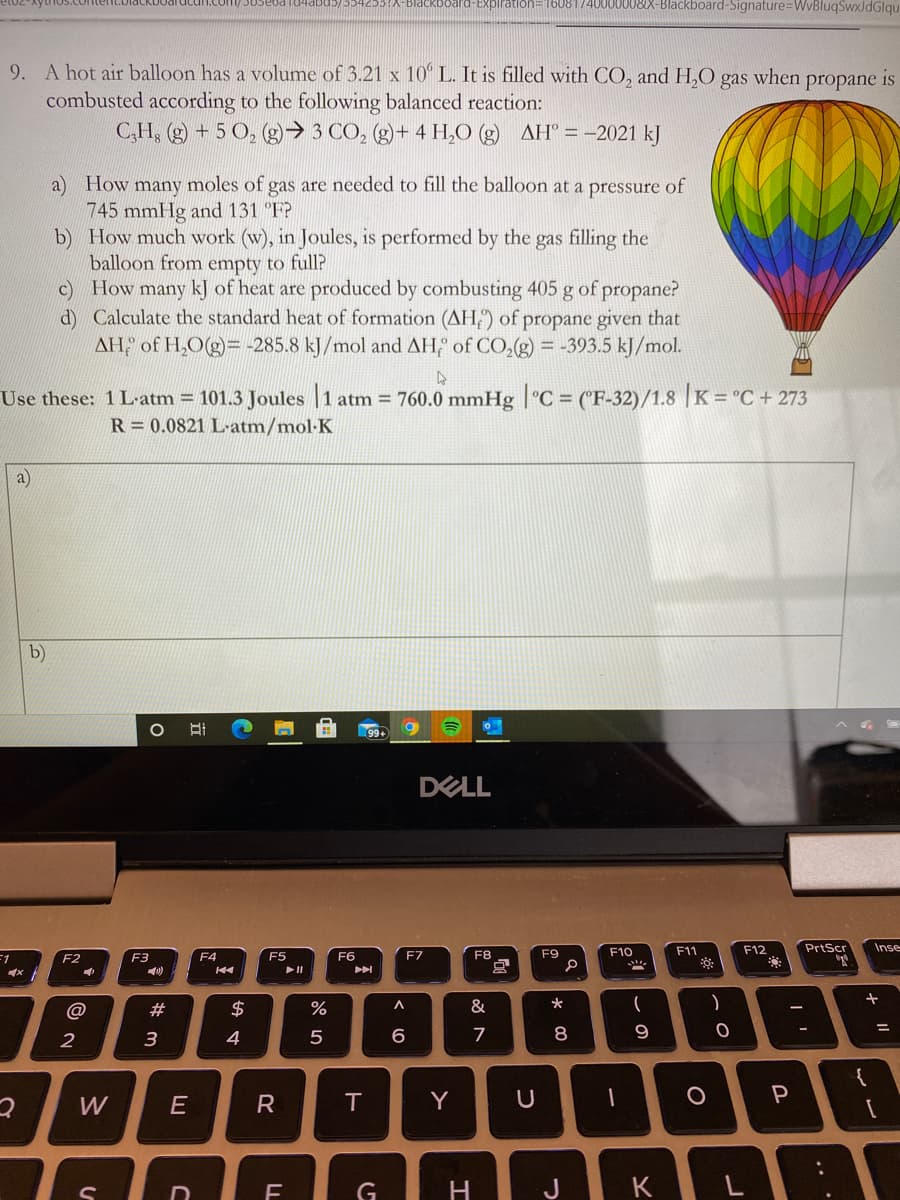
9. A hot air balloon has a volume of 3.21 x 10° L. It is filled with CO, and H,O gas when propane is combusted according to the following balanced reaction: C,H, (g) + 5 O, (g)→ 3 CO, (g)+ 4 H,O (g) AH° = -2021 kJ a) How many moles of gas are needed to fill the balloon at a pressure of 745 mmHg and 131 °F? b) How much work (w), in Joules, is performed by the gas filling the balloon from empty to full? c) How many kJ of heat are produced by combusting 405 g of propane? d) Calculate the standard heat of formation (AH) of propane given that AH, of H,O(g)= -285.8 kJ/mol and AH of CO,(g) = -393.5 kJ/mol. %3D
9. A hot air balloon has a volume of 3.21 x 10° L. It is filled with CO, and H,O gas when propane is combusted according to the following balanced reaction: C,H, (g) + 5 O, (g)→ 3 CO, (g)+ 4 H,O (g) AH° = -2021 kJ a) How many moles of gas are needed to fill the balloon at a pressure of 745 mmHg and 131 °F? b) How much work (w), in Joules, is performed by the gas filling the balloon from empty to full? c) How many kJ of heat are produced by combusting 405 g of propane? d) Calculate the standard heat of formation (AH) of propane given that AH, of H,O(g)= -285.8 kJ/mol and AH of CO,(g) = -393.5 kJ/mol. %3D
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter11: Atomic Mass Spectrometry
Section: Chapter Questions
Problem 11.4QAP
Related questions
Question
I just need help figuring these out.
Transcribed Image Text:16081740000008X-Blackboard-Signature=DWvBluqSwxJdGlqu
9. A hot air balloon has a volume of 3.21 x 10° L. It is filled with CO, and H,O
combusted according to the following balanced reaction:
C,H, (g) + 5 0, (g)→ 3 CO, (g)+ 4 H,O (g) AH° = -2021 kJ
gas
when
propane is
a) How many moles of gas are needed to fill the balloon at a pressure of
745 mmHg and 131 "F?
b) How much work (w), in Joules, is performed by the gas filling the
balloon from empty to full?
c) How many kJ of heat are produced by combusting 405 g of propane?
d) Calculate the standard heat of formation (AH;) of propane given that
AH of H,O(g)= -285.8 kJ/mol and AH, of CO,(g) = -393.5 kJ/mol.
!!
|1
Use these: 1 L-atm = 101.3 Joules
R = 0.0821 L-atm/mol·K
760.0 mmHg |°C = ("F-32)/1.8 |K = °C + 273
atm
a)
b)
9.
DELL
F12
PrtScr
Inse
F3
F5
F6
F7
F8
F9
F10
F1
F2
F4
4)
#
2$
&
2
3
4
5
7
8
9
W
E
Y
D
H

Transcribed Image Text:y NYDM X
E Special X
y A bad X
y! orbital X
y! how to X
A An Eas x
y bad m x
Kathry x
D2-xythos.content.blackboardcdn.com/5b3e6a1d4abd5/354253?X-Blackboard-Expiration316081740000008X-Blackboard-Signature=DW
5. The following quantities are placed in a container: 1.5 x 10 molecules of hydrogen gas
oxygen gas, and 18.0 g of nitrogen gas.
of
a) What is the total mass (in grams) of the mixture?
b) What is the total moles of gas in the mixture?
c) If the container has a total pressure of 828 torr, what is the partial
pressure of each gas (in torr)?
d) If the container has a pressure or 828 torr at 25°C, and can
withstand
the container burst?
pressure of 955 torr, at what temperature (in °C) will
日
99+
DELL
立
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,