9. To prepare a basic buffer of pH=10 how many grams of NH,Cl would you add to 100 ml of 0.1 M NH¸OH ? (K,(NH,OH) =1.84-10³ mol/L; molar mass of NH,Cl = 53.5 g/mol). %3D
9. To prepare a basic buffer of pH=10 how many grams of NH,Cl would you add to 100 ml of 0.1 M NH¸OH ? (K,(NH,OH) =1.84-10³ mol/L; molar mass of NH,Cl = 53.5 g/mol). %3D
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter17: Principles Of Chemical Reactivity: Other Aspects Of Aqueous Equilibria
Section17.2: Controlling Ph: Buffer Solutions
Problem 3RC
Related questions
Question
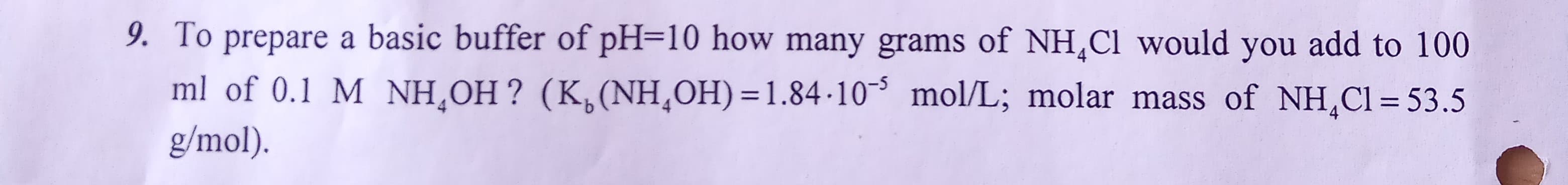
Transcribed Image Text:9. To prepare a basic buffer of pH=10 how many grams of NH,Cl would you add to 100
ml of 0.1 M NH¸OH ? (K,(NH,OH) =1.84-10³ mol/L; molar mass of NH,Cl = 53.5
g/mol).
%3D
Expert Solution
Step 1
The Henderson Hasselbalch equation for basic buffer is given as:
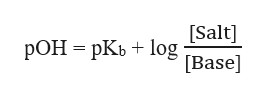
Step 2
Given,
pH basic buffer solution = 10
Volume of solution = 100 mL = 0.1 L (1 mL = 0.001 L)
Molarity of NH4OH (base) = 0.1 M
Kb of NH4OH = 1.84 × 10-5 mol/L = 1.84 × 10-5 M
Molar mass of NH4Cl = 53.5 g/mol
The pOH of the solution and pKb can be calculated as :

Step 3
The concentration of the salt (NH4Cl) can be calculated from Henderson Hasselbalch equation as :

Step by step
Solved in 6 steps with 5 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning