9. You are holding two balloons of the same volume. One balloon contains 1.0 g helium. The other balloon contains neon. What is the mass of neon in the balloon? mol to c O2Smol 1.010 0.20 g b. 1.0 g 4.0 g d. 5.0 g e. 20. g a. Weod 0.25mol dog I mol с. 1
9. You are holding two balloons of the same volume. One balloon contains 1.0 g helium. The other balloon contains neon. What is the mass of neon in the balloon? mol to c O2Smol 1.010 0.20 g b. 1.0 g 4.0 g d. 5.0 g e. 20. g a. Weod 0.25mol dog I mol с. 1
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU4: Toxins: Stoichiometry, Solution Chemistry, And Acids And Bases
SectionU4.11: Mountains Into Molehills: Mass-mole Conversions
Problem 8E
Related questions
Question
When someone in my class asked the professor I'm not sure if she misunderstood and just said yes so please verify.
they asked since mass = volume
he is 1 g so mass of neon would be 1g?
Chegg gave a different answer they said 5. I'm confused
see picture
thx
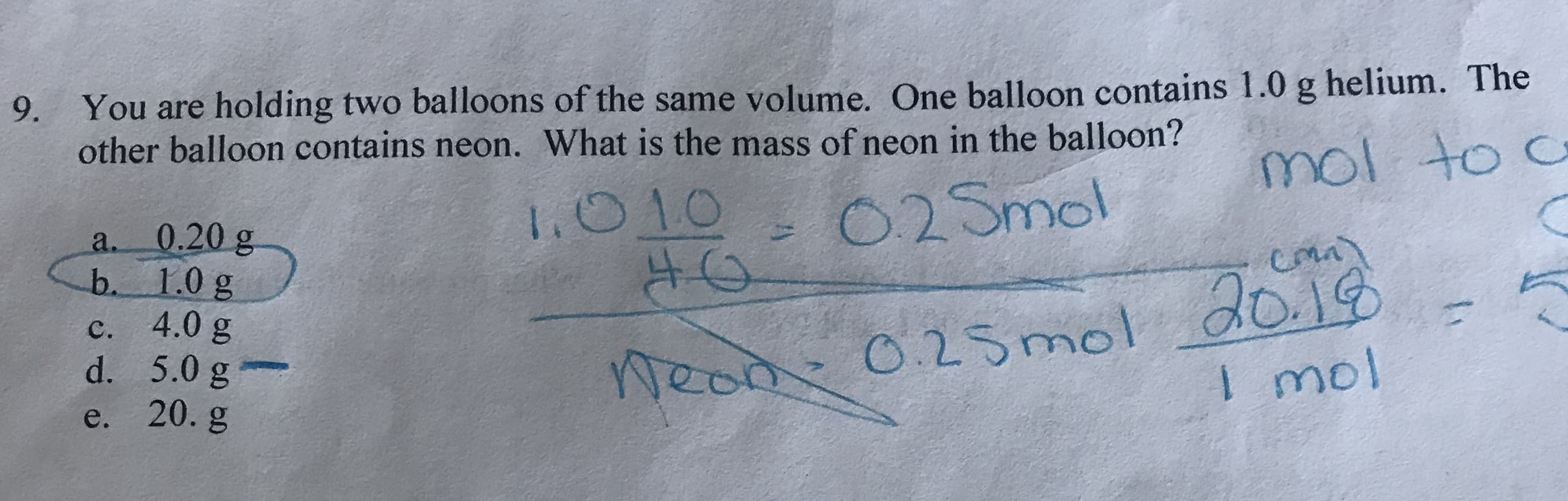
Transcribed Image Text:9. You are holding two balloons of the same volume. One balloon contains 1.0 g helium. The
other balloon contains neon. What is the mass of neon in the balloon?
mol to c
O2Smol
1.010
0.20 g
b. 1.0 g
4.0 g
d. 5.0 g
e. 20. g
a.
Weod 0.25mol dog
I mol
с.
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning