_CH;NH,(g) + _O,(g) → _CO,(g) + _H,0(g) + _N,(g) - Type your answers in all of the blanks and submit Consider the oxidation of methylamine shown in the image above. When properly balanced the coefficient infront of 0, = If you want to combust 45.0 g of CH NH,, how many grams of Oz do you need? oxygen = Type your answer here grams How many grams of N2gas can be formed from this oxidation? nitrogen = Type your answer here grams
_CH;NH,(g) + _O,(g) → _CO,(g) + _H,0(g) + _N,(g) - Type your answers in all of the blanks and submit Consider the oxidation of methylamine shown in the image above. When properly balanced the coefficient infront of 0, = If you want to combust 45.0 g of CH NH,, how many grams of Oz do you need? oxygen = Type your answer here grams How many grams of N2gas can be formed from this oxidation? nitrogen = Type your answer here grams
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 62QAP: Ten mL of concentrated H3PO4 (91.7% by mass, d=1.69g/mL) was accidentally poured into a beaker...
Related questions
Question
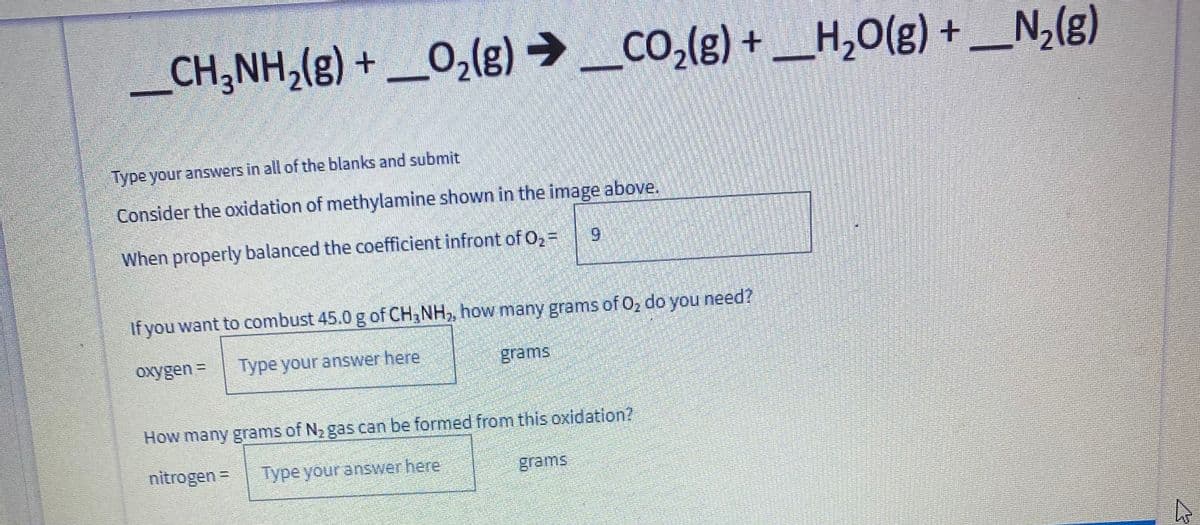
Transcribed Image Text:_CH;NH,(g) + _O,(g) → _co,(g) + _H,O(g) + _N,(g)
H,0(g) +
Type your answers in all of the blanks and submit
Consider the oxidation of methylamine shown in the image above.
When properly balanced the coefficient infront of O, =
6.
If you want to combust 45.0 g of CH3NH,, how many grams of O, do you need?
oxygen =
Type your answer here
grams
How many grams of N2 gas can be formed from this oxidation?
nitrogen =
Type your answer here
grams
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning