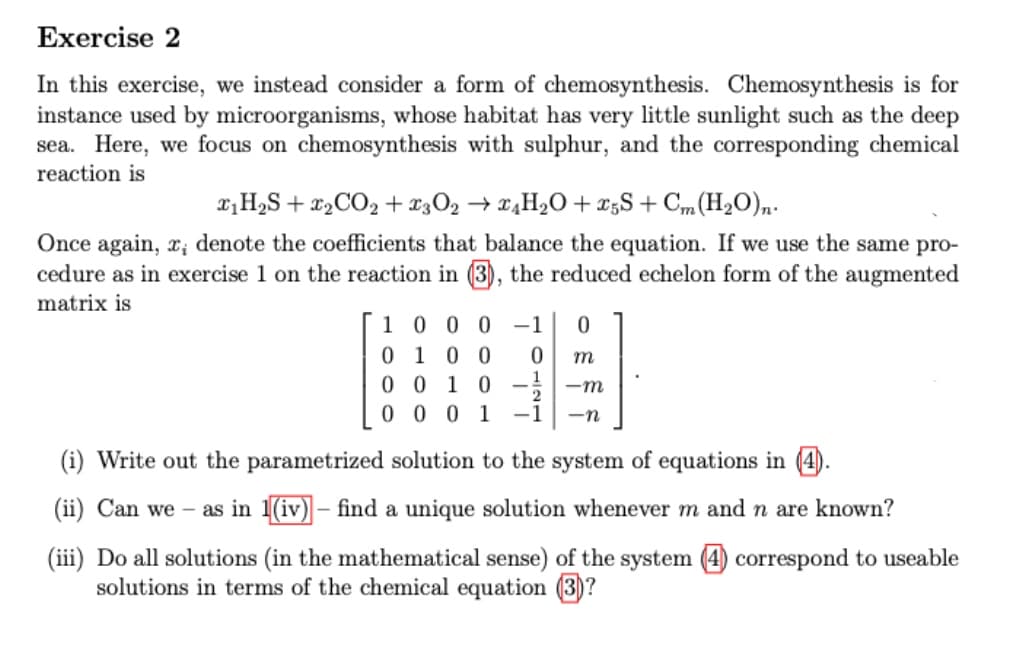
In this exercise, we instead consider a form of chemosynthesis. Chemosynthesis is for instance used by microorganisms, whose habitat has very little sunlight such as the deep sea. Here, we focus on chemosynthesis with sulphur, and the corresponding chemical reaction is ₁H₂S + ₂ CO₂ +£3O₂ → £4H₂O +25S+ Cm (H₂O)n. Once again, x, denote the coefficients that balance the equation. If we use the same pro- cedure as in exercise 1 on the reaction in (3), the reduced echelon form of the augmented matrix is 1000-1 0 0100 m 0010-1 -m 0001-1 -n (i) Write out the parametrized solution to the system of equations in (4). (ii) Can we - as in 1(iv) – find a unique solution whenever m and n are known? (iii) Do all solutions (in the mathematical sense) of the system (4) correspond to useable solutions in terms of the chemical equation (3)? 0
Thermochemistry
Thermochemistry can be considered as a branch of thermodynamics that deals with the connections between warmth, work, and various types of energy, formed because of different synthetic and actual cycles. Thermochemistry describes the energy changes that occur as a result of reactions or chemical changes in a substance.
Exergonic Reaction
The term exergonic is derived from the Greek word in which ‘ergon’ means work and exergonic means ‘work outside’. Exergonic reactions releases work energy. Exergonic reactions are different from exothermic reactions, the one that releases only heat energy during the course of the reaction. So, exothermic reaction is one type of exergonic reaction. Exergonic reaction releases work energy in different forms like heat, light or sound. For example, a glow stick releases light making that an exergonic reaction and not an exothermic reaction since no heat is released. Even endothermic reactions at very high temperature are exergonic.
Step by step
Solved in 2 steps with 2 images




