A 0. 750 g sample of flour was subjected to a Kjeldahl analysis. The ammonia formed after digestion was distilled into 30. 00 mL of 0. 0500 M HCI. The excess HCI was back titrated with 6. 50 mL of 0.0400 M NaOH. Calculate the following parameters in the flour: a. percent N b. percent protein (factor = 5. 70) This problem involves back titration. Solve for the mole of acid reacted with the ammonia. Use the mole of acid to solve the grams of N and protein, use those values to solve the % w/w.
A 0. 750 g sample of flour was subjected to a Kjeldahl analysis. The ammonia formed after digestion was distilled into 30. 00 mL of 0. 0500 M HCI. The excess HCI was back titrated with 6. 50 mL of 0.0400 M NaOH. Calculate the following parameters in the flour: a. percent N b. percent protein (factor = 5. 70) This problem involves back titration. Solve for the mole of acid reacted with the ammonia. Use the mole of acid to solve the grams of N and protein, use those values to solve the % w/w.
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter24: The Standardization Of A Basic Solution And The Determination Of The Molar Mass Of An Acid
Section: Chapter Questions
Problem 3ASA: A 0.3012g sample of an unknown monoprotic acid requires 24.13mL of 0.0944MNaOH for neutralization to...
Related questions
Question
100%
kindly provide complete and full solution. i won't like your solution if it is incomplete or not clear enough to read.
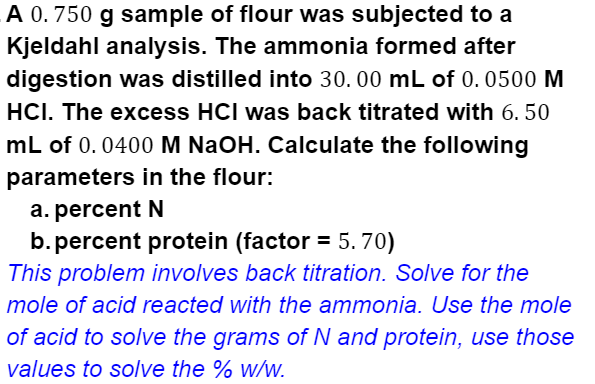
Transcribed Image Text:A 0. 750 g sample of flour was subjected to a
Kjeldahl analysis. The ammonia formed after
digestion was distilled into 30. 00 mL of 0. 0500 M
HCI. The excess HCI was back titrated with 6. 50
mL of 0.0400 M NaOH. Calculate the following
parameters in the flour:
a. percent N
b. percent protein (factor = 5. 70)
This problem involves back titration. Solve for the
mole of acid reacted with the ammonia. Use the mole
of acid to solve the grams of N and protein, use those
values to solve the % w/w.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

