Sulfur containing compound weighing 4.8670 grams was digested and purified to form H,SO¸. A 25.00-mL of 0.0090 M NaOH was added to the collected H SO , and the excess base was then back titrated with 13. 45 mL of 0.0100 M HCI. Calculate the sulfur content in the sample parts per million. This problem involves back titration. Write the two chemical equations involved in the analysis. Identify the analyte, “bridging" reagent and titrant. Solve for the mole of the analyte using the titration values and the stoich relationships. One mole of H,so, is equal to one mole of sulfur in the sample. Solve for the mass of S then ppm S.
Sulfur containing compound weighing 4.8670 grams was digested and purified to form H,SO¸. A 25.00-mL of 0.0090 M NaOH was added to the collected H SO , and the excess base was then back titrated with 13. 45 mL of 0.0100 M HCI. Calculate the sulfur content in the sample parts per million. This problem involves back titration. Write the two chemical equations involved in the analysis. Identify the analyte, “bridging" reagent and titrant. Solve for the mole of the analyte using the titration values and the stoich relationships. One mole of H,so, is equal to one mole of sulfur in the sample. Solve for the mass of S then ppm S.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter15: Acid-base Equilibria
Section: Chapter Questions
Problem 132IP: A 10.00-g sample of the ionic compound NaA, where A is the anion of a weak acid, was dissolved in...
Related questions
Question
100%
kindly provide complete and full solution. I won't like it if it is not clear to read or incomplete.
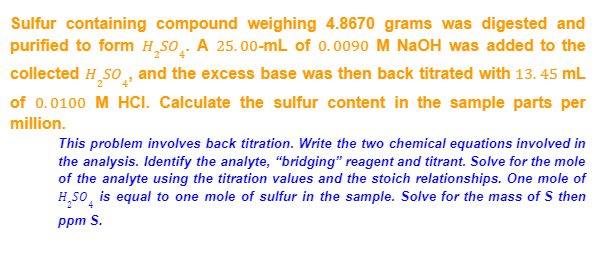
Transcribed Image Text:Sulfur containing compound weighing 4.8670 grams was digested and
purified to form H,SO̟. A 25.00-mL of 0.0090 M NaOH was added to the
collected H,So, and the excess base was then back titrated with 13. 45 mL
of 0.0100 M HCI. Calculate the sulfur content in the sample parts per
million.
This problem involves back titration. Write the two chemical equations involved in
the analysis. Identify the analyte, "bridging" reagent and titrant. Solve for the mole
of the analyte using the titration values and the stoich relationships. One mole of
H,So, is equal to one mole of sulfur in the sample. Solve for the mass of S then
ppm S.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning