A 0.448 g sample of propanoic acid (CH3CH2COOH, Ka = 1.3. x 10-5) was dissolved to a final volume of 50.0 cm3. This solution %3D underwent titration with 0.16 M NaOH. Calculate the pH of solution at the equivalence point.
A 0.448 g sample of propanoic acid (CH3CH2COOH, Ka = 1.3. x 10-5) was dissolved to a final volume of 50.0 cm3. This solution %3D underwent titration with 0.16 M NaOH. Calculate the pH of solution at the equivalence point.
Chapter14: Principles Of Neutralization Titrations
Section: Chapter Questions
Problem 14.38QAP
Related questions
Question
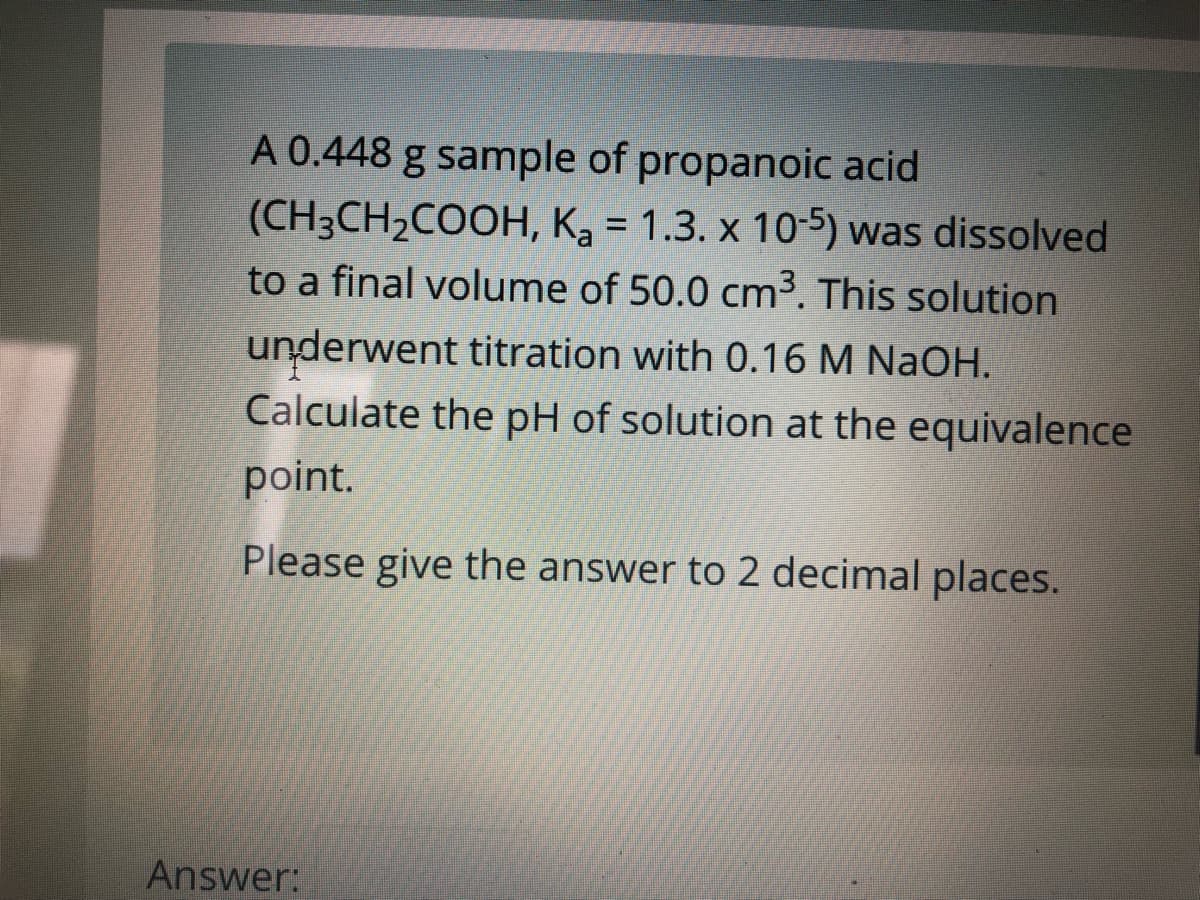
Transcribed Image Text:A 0.448 g sample of propanoic acid
(CH3CH2COOH, Ka = 1.3. x 105) was dissolved
to a final volume of 50.0 cm³. This solution
%3D
underwent titration with 0.16 M NaOH.
Calculate the pH of solution at the equivalence
point.
Please give the answer to 2 decimal places.
Answer:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning