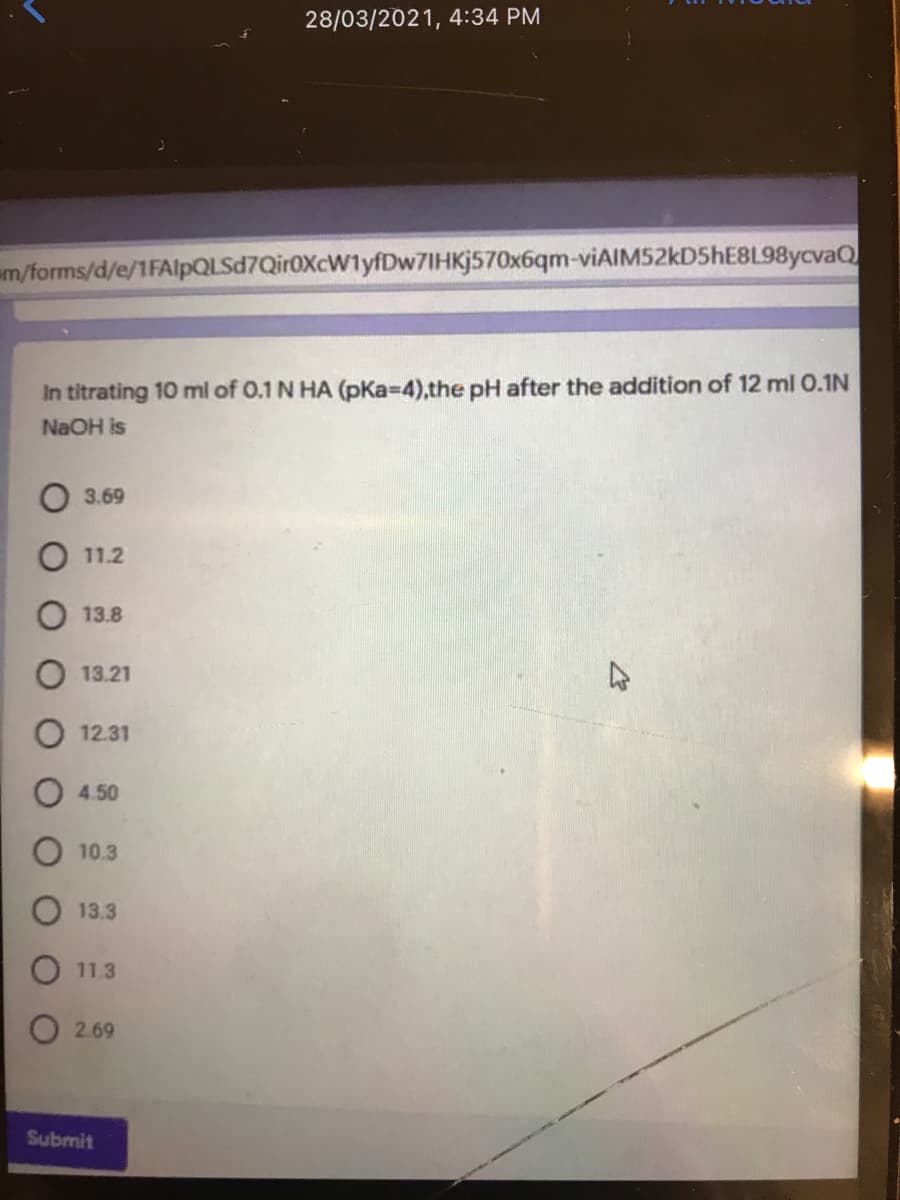
In titrating 10 ml of 0.1 N HA (pKa-4),the pH after the addition of 12 ml 0.1N NaOH is O 3.69 О 11.2 13.8 13.21 12.31 O 4.50 О 10.3 O 13.3 O 11.3 O 2.69
In titrating 10 ml of 0.1 N HA (pKa-4),the pH after the addition of 12 ml 0.1N NaOH is O 3.69 О 11.2 13.8 13.21 12.31 O 4.50 О 10.3 O 13.3 O 11.3 O 2.69
Chapter15: Complex Acid/base Systems
Section: Chapter Questions
Problem 15.29QAP
Related questions
Question
Transcribed Image Text:28/03/2021, 4:34 PM
am/forms/d/e/1FAlpQLSd7Qir0XcW1yfDw7IHKj570x6qm-viAIM52kD5hE8L98ycvaQ
In titrating 10 ml of 0.1 N HA (pKa-D4),the pH after the addition of 12 ml 0.1N
NaOH is
3.69
11.2
13.8
13.21
12.31
4.50
10.3
13.3
11.3
O 2.69
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole