A 1.0-kg bar of copper is heated at atmospheric pressure so that its temperature increases from 20°C to 50°C. (A) What is the work done on the copper bar by the surrounding atmosphere? (B) How much energy is transferred to the copper bar by heat? (C) What is the increase in internal energy of the copper bar?
A 1.0-kg bar of copper is heated at atmospheric pressure so that its temperature increases from 20°C to 50°C. (A) What is the work done on the copper bar by the surrounding atmosphere? (B) How much energy is transferred to the copper bar by heat? (C) What is the increase in internal energy of the copper bar?
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter1: Basic Modes Of Heat Transfer
Section: Chapter Questions
Problem 1.48P: A horizontal, 3-mm-thick flat-copper plate, 1-m long and 0.5-m wide, is exposed in air at 27C to...
Related questions
Question
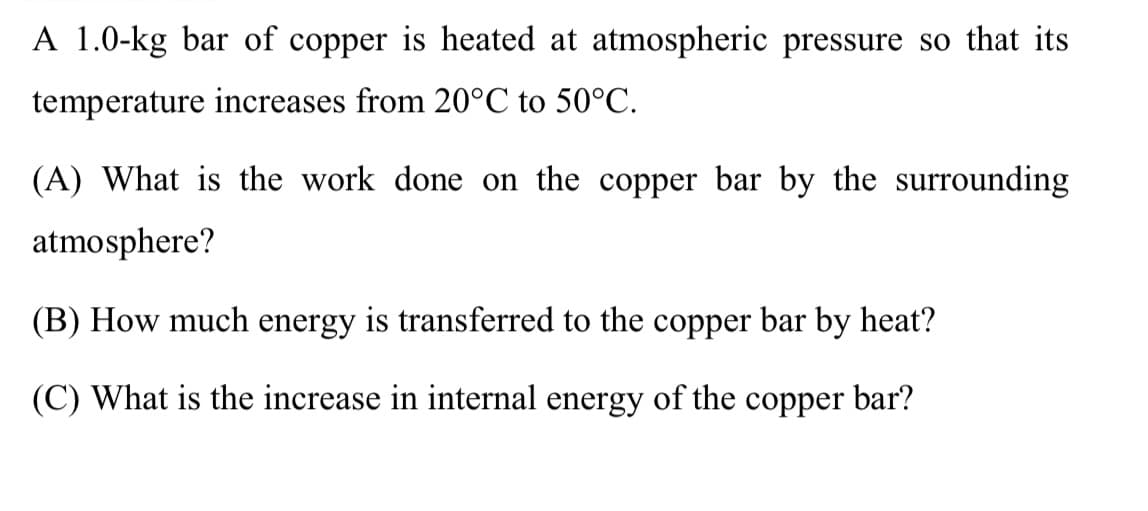
Transcribed Image Text:A 1.0-kg bar of copper is heated at atmospheric pressure so that its
temperature increases from 20°C to 50°C.
(A) What is the work done on the copper bar by the surrounding
atmosphere?
(B) How much energy is transferred to the copper bar by heat?
(C) What is the increase in internal energy of the copper bar?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning