A 1.00 L rigid cylinder contains one mole of neon gas at 25 °C. Calculate the pressure using (a) the ideal gas law, and (b) the van der Waals equation (c) A much more accurate equation is the virial equation, В (Т) + Vm С (Т) pVm 1+ RT where B(T) and C(T) and C(T) are 11.42 cm3 mol1 and 221 cm6 mol2, respectively. Assuming the pressure calculated using the virial equation is the "true" value, determine the percent error by using ideal gas law and van der Waals equation are functions of temperature. For neon at 25°C, the values of B(T)
A 1.00 L rigid cylinder contains one mole of neon gas at 25 °C. Calculate the pressure using (a) the ideal gas law, and (b) the van der Waals equation (c) A much more accurate equation is the virial equation, В (Т) + Vm С (Т) pVm 1+ RT where B(T) and C(T) and C(T) are 11.42 cm3 mol1 and 221 cm6 mol2, respectively. Assuming the pressure calculated using the virial equation is the "true" value, determine the percent error by using ideal gas law and van der Waals equation are functions of temperature. For neon at 25°C, the values of B(T)
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter9: Gases
Section: Chapter Questions
Problem 104E: A 0.245-L flask contains 0.467 mol CO2 at 159 C. Calculate the pressure: (a) using the ideal gas law...
Related questions
Question
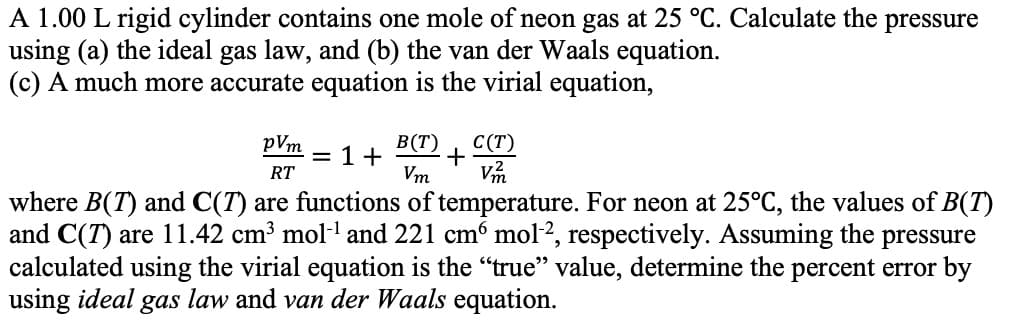
Transcribed Image Text:A 1.00 L rigid cylinder contains one mole of neon gas at 25 °C. Calculate the pressure
using (a) the ideal gas law, and (b) the van der Waals equation
(c) A much more accurate equation is the virial equation,
В (Т)
+
Vm
С (Т)
pVm
1+
RT
where B(T) and C(T)
and C(T) are 11.42 cm3 mol1 and 221 cm6 mol2, respectively. Assuming the pressure
calculated using the virial equation is the "true" value, determine the percent error by
using ideal gas law and van der Waals equation
are functions of temperature. For neon at 25°C, the values of B(T)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 5 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning