A 1.25 kg piece of unknown metal is boiled. The metal is then soaked in 500 g of water at 20 ° C. If the final temperature of the mixture is 30 degrees C, what is the heat capacity of the metal? Can you identify this metal using the following table.
A 1.25 kg piece of unknown metal is boiled. The metal is then soaked in 500 g of water at 20 ° C. If the final temperature of the mixture is 30 degrees C, what is the heat capacity of the metal? Can you identify this metal using the following table.
Chapter2: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 93AP: Unreasonable results. (a) Find the sped of hydrogen sulfide, H2S, molecules at a temperature of 250...
Related questions
Question
A 1.25 kg piece of unknown metal is boiled. The metal is then soaked in 500 g
of water at 20 ° C. If the final temperature of the mixture is 30 degrees C, what is the heat capacity of the metal? Can you identify this metal using the following table.
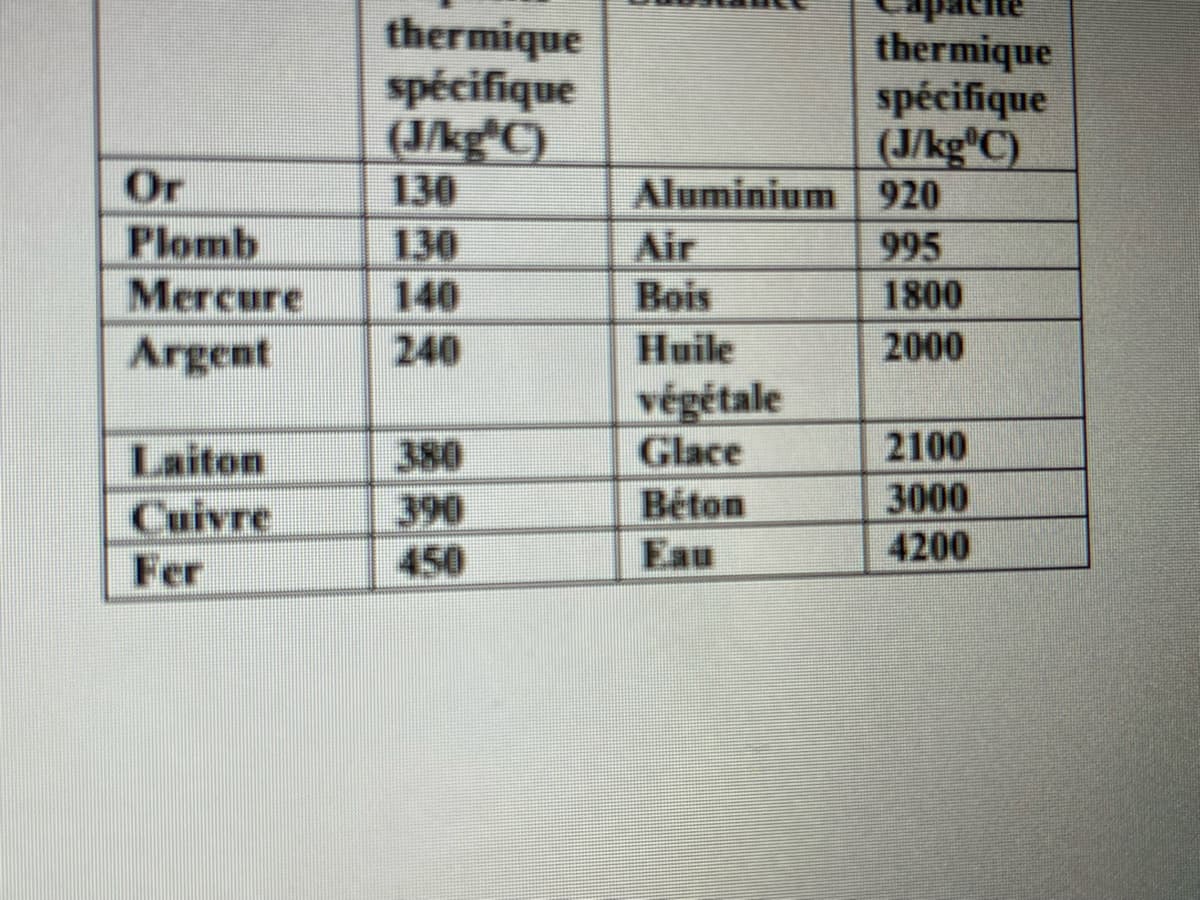
Transcribed Image Text:thermique
spécifique
(J/kg*C)
130
thermique
spécifique
(J/kg C)
Aluminium | 920
Or
Plomb
Mercure
130
Air
995
140
Bois
1800
Argent
240
Huile
2000
végétale
Glace
Béton
Laiton
380
2100
3000
4200
390
Cuivre
Fer
450
Eau
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

