In a laboratory experiment, 16.0 J of heat raised the temperature of a 199-g object from 25.0° C to 35.0° C. What is the specific heat of the object? O 1600 J/kg • K O 0.00120 J/kg • K O 8.04 J/kg • K O 3.18 x 106 J/kg • K
In a laboratory experiment, 16.0 J of heat raised the temperature of a 199-g object from 25.0° C to 35.0° C. What is the specific heat of the object? O 1600 J/kg • K O 0.00120 J/kg • K O 8.04 J/kg • K O 3.18 x 106 J/kg • K
Chapter1: Temperature And Heat
Section: Chapter Questions
Problem 119AP: For the human body, what is the rate of heat transfer by conduction through the body's tissue with...
Related questions
Question
number 25 and 26 pls help :((
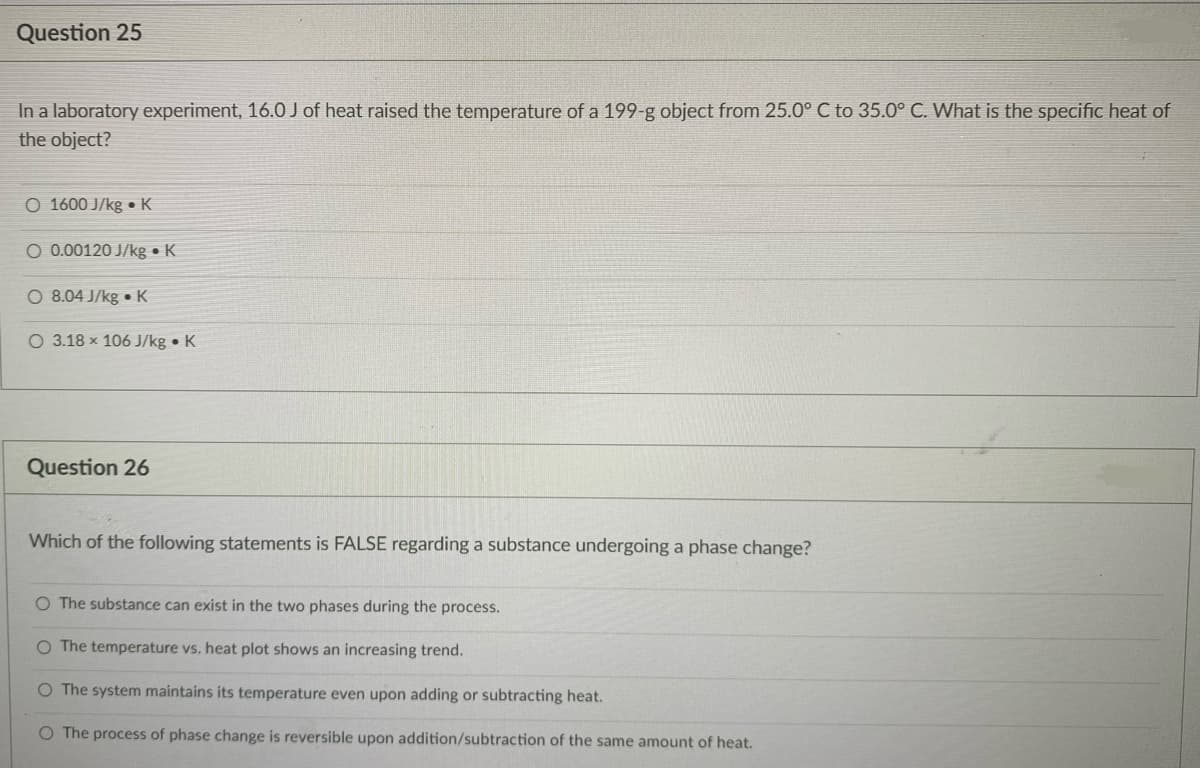
Transcribed Image Text:Question 25
In a laboratory experiment, 16.0 J of heat raised the temperature of a 199-g object from 25.0° C to 35.0° C. What is the specific heat of
the object?
O 1600 J/kg • K
O 0.00120 J/kg • K
O 8.04 J/kg • K
O 3.18 x 106 J/kg • K
Question 26
Which of the following statements is FALSE regarding a substance undergoing a phase change?
O The substance can exist in the two phases during the process.
O The temperature vs. heat plot shows an increasing trend.
O The system maintains its temperature even upon adding or subtracting heat.
O The process of phase change is reversible upon addition/subtraction of the same amount of heat.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College