A 10.00 L tank at -7.0 °C is filled with 4.65 g of sulfur hexafluoride gas and 3.91g of dinitrogen monoxide gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction of each gas. Round each of your answers to 3 significant digits. gas mole fraction sulfur hexafluoride dinitrogen monoxide
A 10.00 L tank at -7.0 °C is filled with 4.65 g of sulfur hexafluoride gas and 3.91g of dinitrogen monoxide gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction of each gas. Round each of your answers to 3 significant digits. gas mole fraction sulfur hexafluoride dinitrogen monoxide
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section: Chapter Questions
Problem 67QRT
Related questions
Question
100%
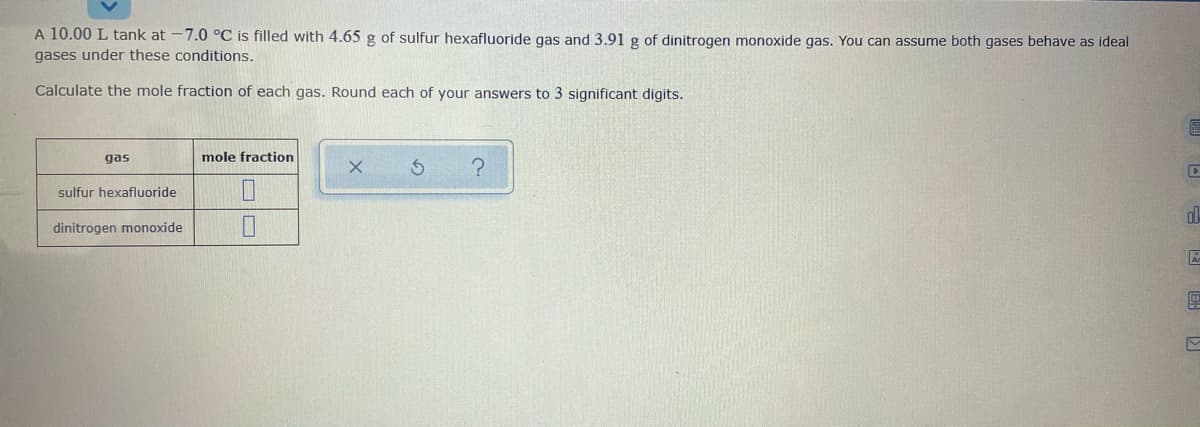
Transcribed Image Text:A 10.00 L tank at -7.0 °C is filled with 4.65 g of sulfur hexafluoride gas and 3.91 g of dinitrogen monoxide gas. You can assume both gases behave as ideal
gases under these conditions.
Calculate the mole fraction of each gas. Round each of your answers to 3 significant digits.
gas
mole fraction
sulfur hexafluoride
dinitrogen monoxide
国 同 同 回
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning