A 100 mL solution of 0.1 M amino acid (AA) at pH 1.0 was titrated with 1.0 M NaOH solution. The pH was monitored, and the results were plotted on a graph, as shown below. The key points in the titration are I to VII. designated 12 10 -9.7 817 pH 6 4 2 3.9. 2.0 0 1 0.5 1 IV V VI 1.5 2 2.5 Equivalents of OH 3 VII
A 100 mL solution of 0.1 M amino acid (AA) at pH 1.0 was titrated with 1.0 M NaOH solution. The pH was monitored, and the results were plotted on a graph, as shown below. The key points in the titration are I to VII. designated 12 10 -9.7 817 pH 6 4 2 3.9. 2.0 0 1 0.5 1 IV V VI 1.5 2 2.5 Equivalents of OH 3 VII
Chapter10: Reconstitution Of Powdered Drugs
Section: Chapter Questions
Problem 19SST
Related questions
Question
Snwer question 6
![6. Region/point where the solution has a 50:50 percent mixture of the (0) and the (-1) species.
[Select ]
[ Select ]
VII
VI
IV
V
II
Next >
II](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Ff03e3e3e-dbe3-4ed0-b094-53a4b5e345c9%2F5121234a-6934-4059-aae8-3a22f964d723%2Fclzej1j_processed.jpeg&w=3840&q=75)
Transcribed Image Text:6. Region/point where the solution has a 50:50 percent mixture of the (0) and the (-1) species.
[Select ]
[ Select ]
VII
VI
IV
V
II
Next >
II
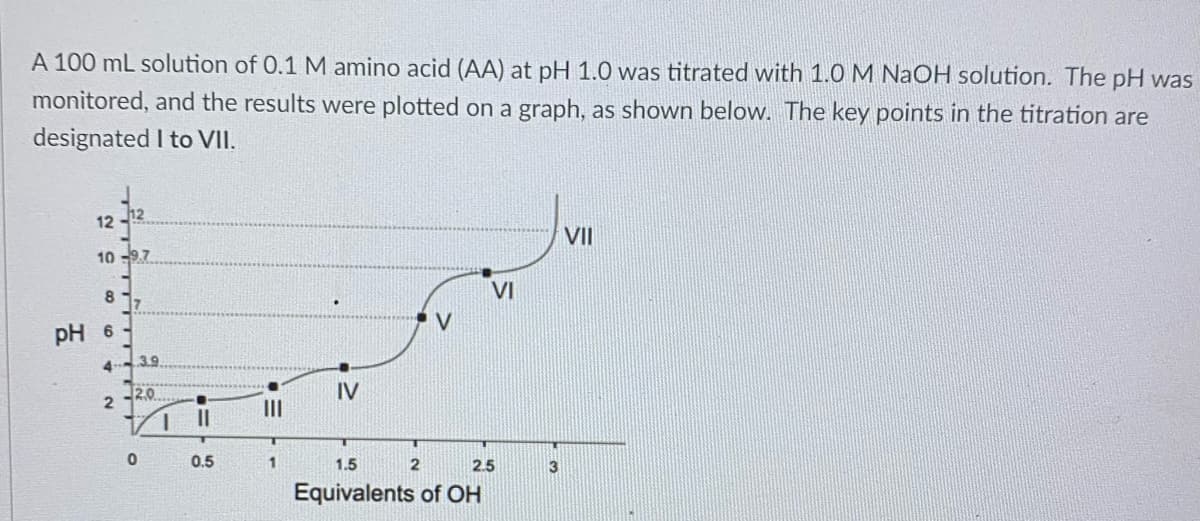
Transcribed Image Text:A 100 mL solution of 0.1 M amino acid (AA) at pH 1.0 was titrated with 1.0 M NaOH solution. The pH was
monitored, and the results were plotted on a graph, as shown below. The key points in the titration are
designated I to VII.
12
VII
10 -9.7
VI
17
V
pH 6
3.9
4
12.0
IV
II
1.
0.5
1.5
2.5
3
Equivalents of OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you



