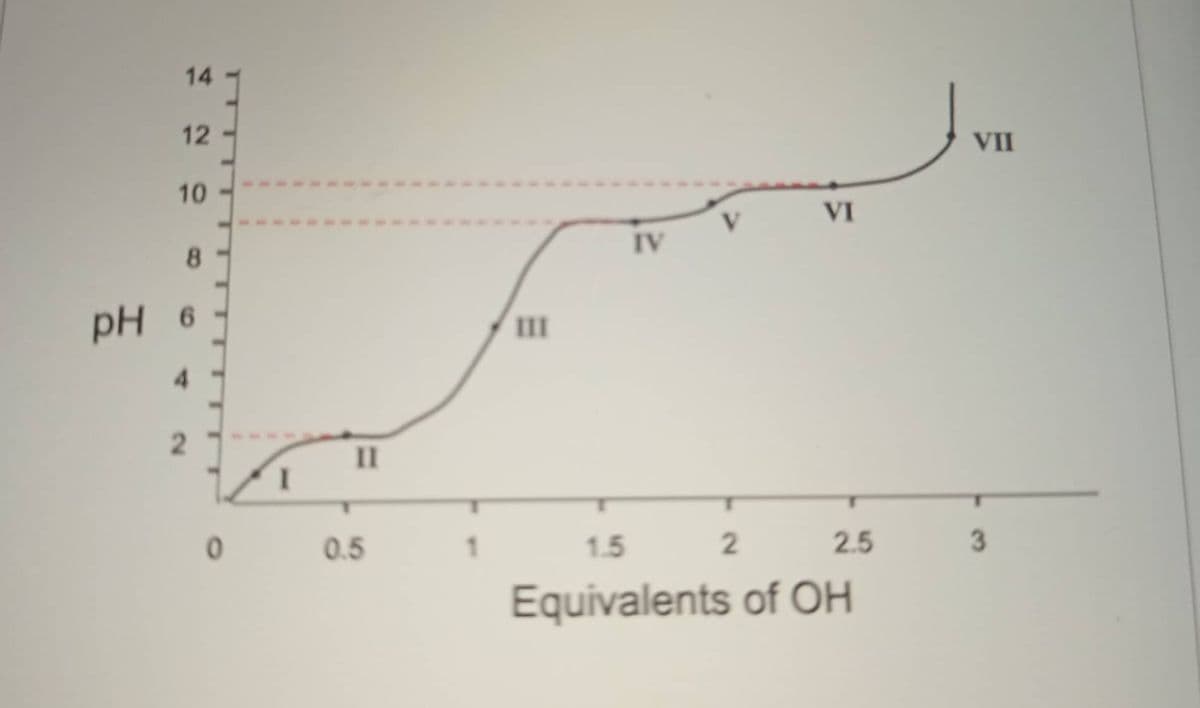
A 100 ml solution of 0.1 M amino acid (AA) at ph 1.0 was titrated with NaOH solution. The pH was monitored, and the results were plotted on the graph. The keypoints in the titration are designated I to VII. What is the possible identity of the amino acid? What is the isoelectric point of AA? what is the pKa corresponding to the dissociation of the alpha carboxylic group?
A 100 ml solution of 0.1 M amino acid (AA) at ph 1.0 was titrated with NaOH solution. The pH was monitored, and the results were plotted on the graph. The keypoints in the titration are designated I to VII.
What is the possible identity of the amino acid?
What is the isoelectric point of AA?
what is the pKa corresponding to the dissociation of the alpha carboxylic group?
Region/point where AA is predominantly present as a (-1) charged species?
The effective buffering range for the amino acid in the acidic region?
Region/point where the solution has 50:50 percent mixture of the (0) and (-1) species
Step by step
Solved in 4 steps

Region/point where AA is predominantly present as a (-1) charged species?
The effective buffering range for the amino acid in the acidic region?
Region/point where the solution has 50:50 percent mixture of the (0) and (-1) species







