A 12.63 g sample of calcium ore was dissolved in HCI and gravimetrically analyzed, through the precipitation of calcium into CaC204 H20. The precipitate was filtered, washed, dried, and ignited at 500 °C until the weight was constant, giving a final mass of 2.35 grams pure CaCO3 (100.087 g/mol). Calculate the % Calcium (40.078 g/mol) in the sample. O 16.7% O 7.45% O 15.4% O 33.4%
A 12.63 g sample of calcium ore was dissolved in HCI and gravimetrically analyzed, through the precipitation of calcium into CaC204 H20. The precipitate was filtered, washed, dried, and ignited at 500 °C until the weight was constant, giving a final mass of 2.35 grams pure CaCO3 (100.087 g/mol). Calculate the % Calcium (40.078 g/mol) in the sample. O 16.7% O 7.45% O 15.4% O 33.4%
Chapter79: Solubility
Section: Chapter Questions
Problem 1P
Related questions
Question
36 and 38
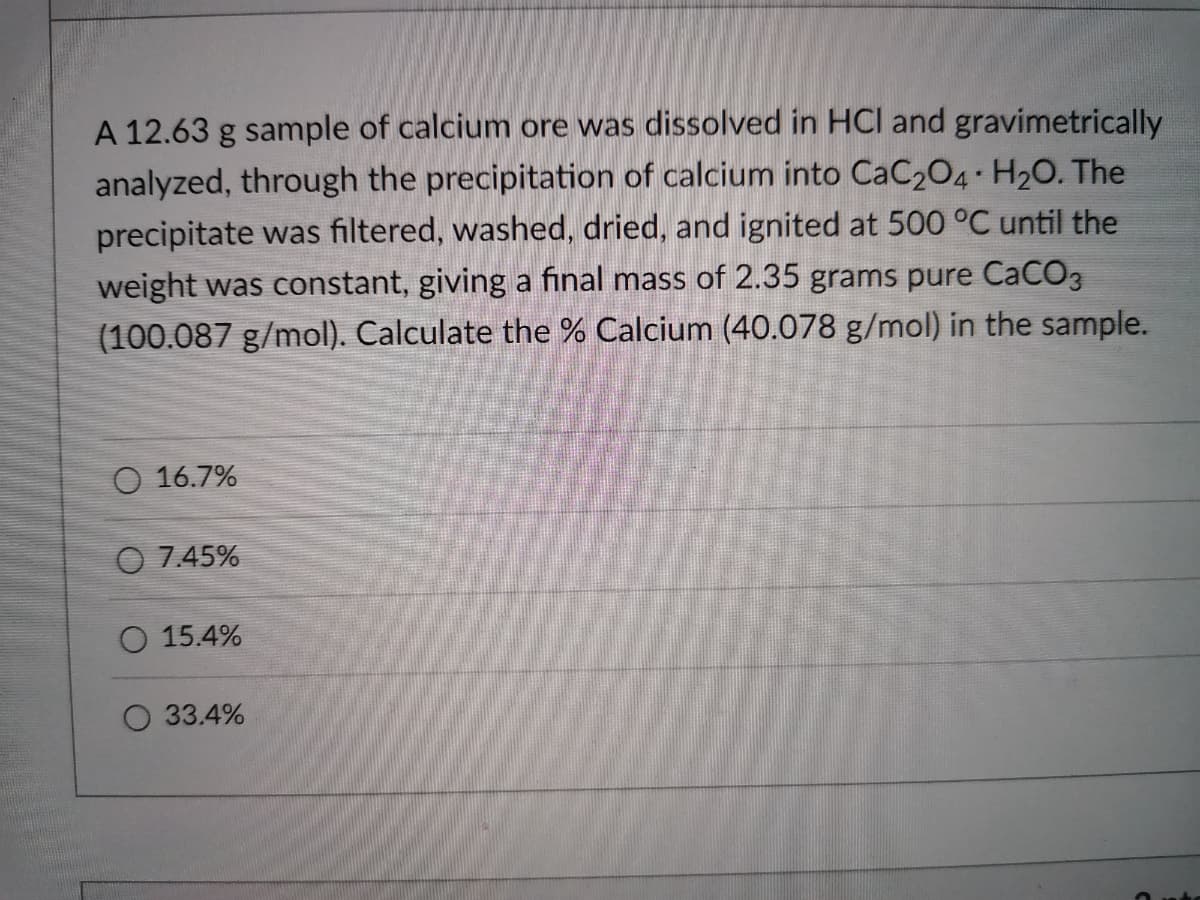
Transcribed Image Text:A 12.63 g sample of calcium ore was dissolved in HCI and gravimetrically
analyzed, through the precipitation of calcium into CaC204 H20. The
precipitate was filtered, washed, dried, and ignited at 500 °C until the
weight was constant, giving a final mass of 2.35 grams pure CaCO3
(100.087 g/mol). Calculate the % Calcium (40.078 g/mol) in the sample.
O 16.7%
O 7.45%
O 15.4%
33.4%

Transcribed Image Text:A 5.70 g iron ore was dissolved in concentrated HCI. After the dissolution,
the resulting solution was made basic by adding 6.0 M NAOH slowly until
the solution is slightly turbid. Urea was then added and the solution was
heated for 3 hours. The precipitate was filtered using an ashless filter paper
and ignited to form Fe2O3 (159.69 g/mol). The mass of precipitate is 2.1 g.
Find the % FegO4 (231.54 g/mol) in the iron ore sample.
О 33.4%
О 34.0%
O 37.7%
O 35.6%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax
