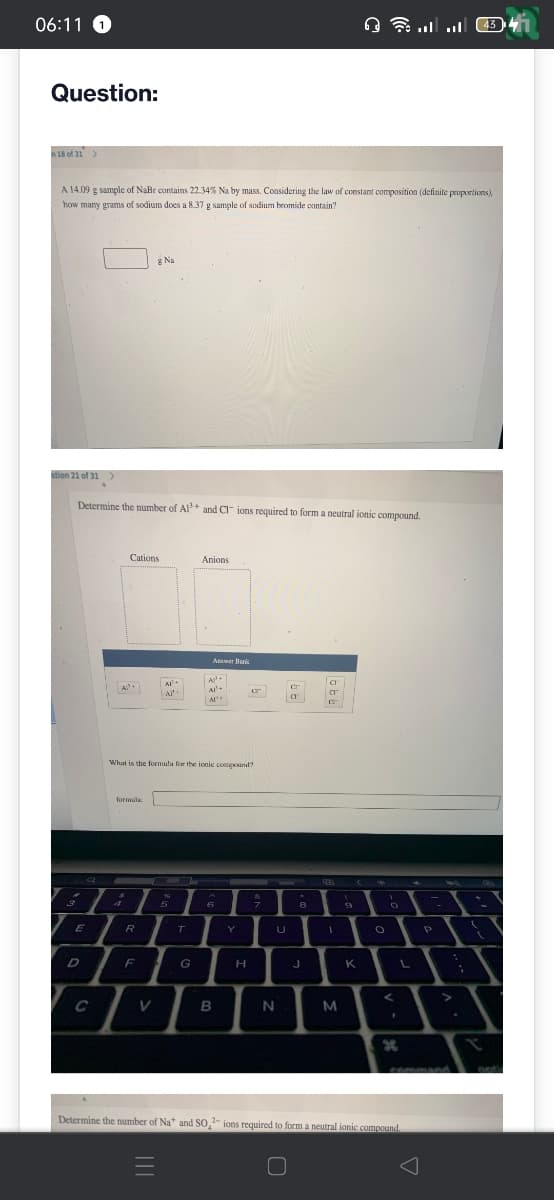
A 14.09 g sample of NaBr contains 22.34% Na by mass. Considering the law of constant composition (definite proportions), how many grams of sodium does a 8.37 g sample of sodium bromide contain? stion 21 of 31 g Na Determine the number of Al³+ and CI ions required to form a neutral ionic compound. Cations Al Al Anions Anwer Banic A²+ Al- AP UT What is the formula for the ionic compound? 99 666
A 14.09 g sample of NaBr contains 22.34% Na by mass. Considering the law of constant composition (definite proportions), how many grams of sodium does a 8.37 g sample of sodium bromide contain? stion 21 of 31 g Na Determine the number of Al³+ and CI ions required to form a neutral ionic compound. Cations Al Al Anions Anwer Banic A²+ Al- AP UT What is the formula for the ionic compound? 99 666
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter12: Chemical Bonding
Section: Chapter Questions
Problem 103AP
Related questions
Question
Please send me the question in 20 minutes it's very urgent plz
Transcribed Image Text:06:11 1
Question:
n 18 of 31 >
A 14.09 g sample of NaBr contains 22.34% Na by mass. Considering the law of constant composition (definite proportions),
how many grams of sodium does a 8.37 g sample of sodium bromide contain?
stion 21 of 31
3
Determine the number of Al³+ and CI ions required to form a neutral ionic compound.
E
D
C
Al
Cations
formala
$
4
R
g Na
F
V
What is the formula for the ionic compound?
Al³+
AT
=
5
Tue
T
Anions
G
Answer Bank
A+
Al²+
Al'
6
B
Y
C
H
7
U
N
cr
a
8
J
Cr
Cr
Cr
1
M
9
K
O
>
<
H
Determine the number of Na* and SO,2- ions required to form a neutral ionic compound.
P
command
A
2
:
4371
D
.
(
not
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning