A 15 mL sample of water from the lemur exhibit required 3.95 mL of a sulfuric acid titrant to reacht he endpoint. Calculate the alkalinity of the exhibit in mg/L of CaCO3 if the titrant conentration was 0.017 M. The molecular weight of calcium carbonate is 100.0869 g/mol. CaCO3(aq) + H2SO4(aq) --> CaSO4(aq) + H2O(1) + CO2(g)
A 15 mL sample of water from the lemur exhibit required 3.95 mL of a sulfuric acid titrant to reacht he endpoint. Calculate the alkalinity of the exhibit in mg/L of CaCO3 if the titrant conentration was 0.017 M. The molecular weight of calcium carbonate is 100.0869 g/mol. CaCO3(aq) + H2SO4(aq) --> CaSO4(aq) + H2O(1) + CO2(g)
Chapter17: Complexation And Precipitation Reactions And Titrations
Section: Chapter Questions
Problem 17.33QAP
Related questions
Question
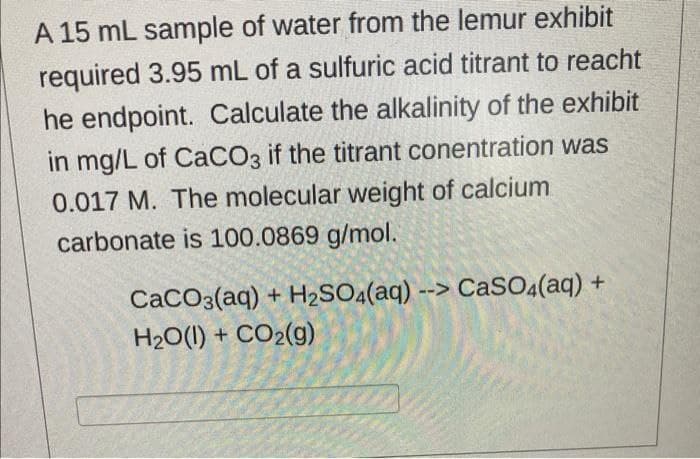
Transcribed Image Text:A 15 mL sample of water from the lemur exhibit
required 3.95 mL of a sulfuric acid titrant to reacht
he endpoint. Calculate the alkalinity of the exhibit
in mg/L of CaCO3 if the titrant conentration was
0.017 M. The molecular weight of calcium
carbonate is 100.0869 g/mol.
CaCO3(aq) + H2SO4(aq) --> CaSO.(aq) +
H20(1) + CO2(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

