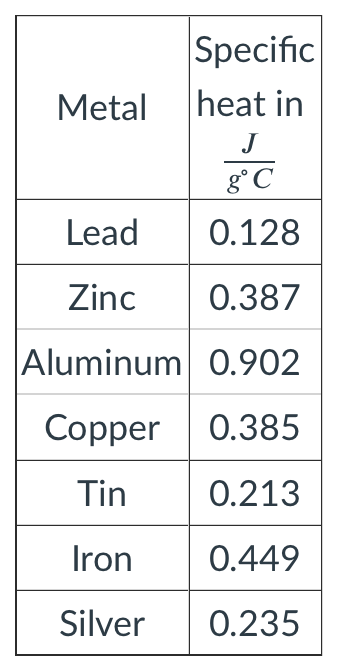
A 19.7724 grams of metal was heated to 100.001∘C when the metal was placed into 155.00 grams of water at 23.721∘C the temperature rose to 24.213∘C. What is the specific heat of this metal? What is the identity of this metal?
A 19.7724 grams of metal was heated to 100.001∘C when the metal was placed into 155.00 grams of water at 23.721∘C the temperature rose to 24.213∘C. What is the specific heat of this metal? What is the identity of this metal?
Chapter2: Basic Statistical Analysis With Excel
Section: Chapter Questions
Problem 12P
Related questions
Question
A 19.7724 grams of metal was heated to 100.001∘C when the metal was placed into 155.00 grams of water at 23.721∘C the temperature rose to 24.213∘C. What is the specific heat of this metal? What is the identity of this metal?
Transcribed Image Text:Specific
heat in
Metal
J
g° C
Lead
0.128
Zinc
0.387
Aluminum 0.902
Copper 0.385
Tin
0.213
Iron
0.449
Silver
0.235
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning