A 20.0 g sample of KOH (A)(MW = 56.10 g A) will react with what volume (in mL) of 0.500 M KH2P04 (s) (B)? Use A and B to show compounds in the working. Given: KH2PO4 (s) + 2 KOH (aq) ----> K3PO4 (aq) + 2 H20 (1) 1. 20.0 g A x 2. 3. 5. 7. 8. 9. 4. 6.
A 20.0 g sample of KOH (A)(MW = 56.10 g A) will react with what volume (in mL) of 0.500 M KH2P04 (s) (B)? Use A and B to show compounds in the working. Given: KH2PO4 (s) + 2 KOH (aq) ----> K3PO4 (aq) + 2 H20 (1) 1. 20.0 g A x 2. 3. 5. 7. 8. 9. 4. 6.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter21: Chemistry Of The Nonmetals
Section: Chapter Questions
Problem 42QAP
Related questions
Question
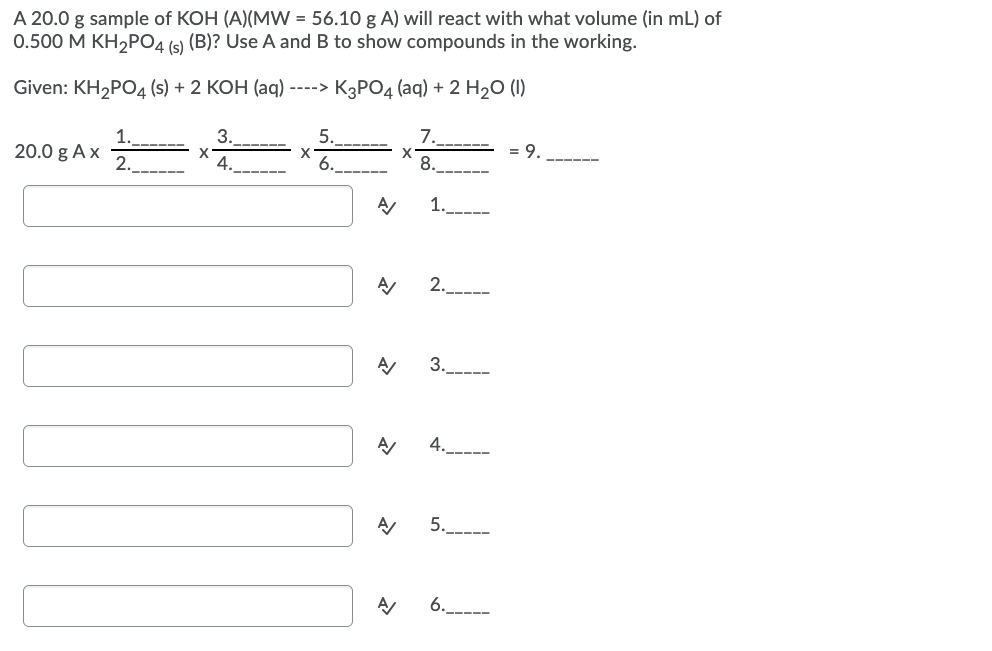
Transcribed Image Text:A 20.0 g sample of KOH (A)(MW = 56.10 g A) will react with what volume (in mL) of
0.500 M KH,PO4 (s) (B)? Use A and B to show compounds in the working.
Given: KH2POД (s) + 2 КОН (aq) ----» КзРОд (аq) + 2 H20 ()
1.
3.
5.
7.
20.0 g Ax
2.
4.----
= 9.
---- --
6.
8.
1.
2.
3.
4.
5.----
6.__---
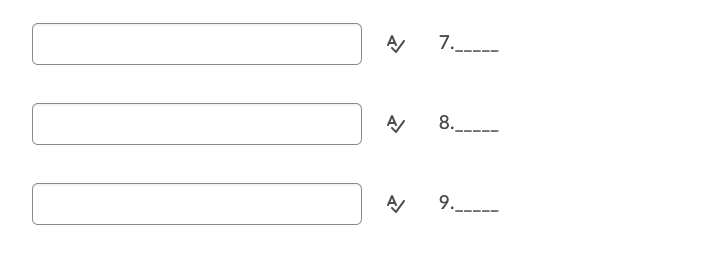
Transcribed Image Text:7.____
8.
___.
9.--
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning