A 25.00 mL solution was prepared by dissolving CaCO3 solid. It required 24.25 mL of a standard 0.02 M EDTA (ethylenediaminetetraacetic acid) solution to titrate the Ca2+ ions to the end point. a. How many moles of EDTA are contained in the 24.25 mL used for tritration. b. How many moles of CaCO3 were used? c. What is the concentration (molarity) of Ca2+ in the 25.00 mL of solution?
A 25.00 mL solution was prepared by dissolving CaCO3 solid. It required 24.25 mL of a standard 0.02 M EDTA (ethylenediaminetetraacetic acid) solution to titrate the Ca2+ ions to the end point.
a. How many moles of EDTA are contained in the 24.25 mL used for tritration.
b. How many moles of CaCO3 were used?
c. What is the concentration (molarity) of Ca2+ in the 25.00 mL of solution?
Solution stoichiometry is mainly based on the calculation of moles and volumes. These two values are used to calculate the molarity of solution. The relation between moles, volume and molarity is as given below;
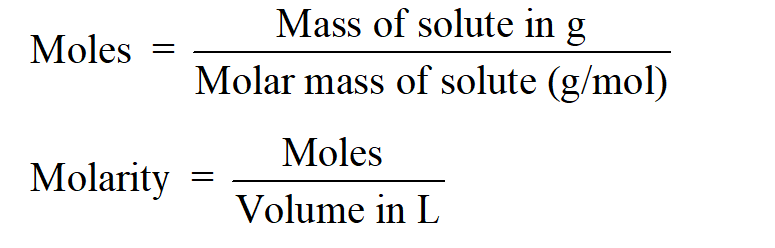
In the chemistry analysis, the calcium ions can be analyzed with the help of EDTA in the presence of an indicator. Here EDTA stands for ethylene diamine tetraacetic acid and can be formulated as H4C10H12N2O4. Since EDTA is not very water soluble therefore the sodium salt is used, Na2H2C10H12N2O4.
The balance chemical equation of EDTA with calcium ions can be written as:
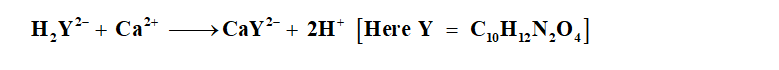
Step by step
Solved in 6 steps with 4 images


