A 250 cm volumetric flask contains exactly 200,0 cm3 of a 0,025 mol.dm sulphuric acid solution. Thereafter ten (10) sodium hydroxide pellets, each of mass 0,1 g are dropped into the flask. After the pellets have dissolved completely, the flask is topped to the 250 cm3 mark with water and the contents are thoroughly homogenised. Determine the pH of the resulting solution.
A 250 cm volumetric flask contains exactly 200,0 cm3 of a 0,025 mol.dm sulphuric acid solution. Thereafter ten (10) sodium hydroxide pellets, each of mass 0,1 g are dropped into the flask. After the pellets have dissolved completely, the flask is topped to the 250 cm3 mark with water and the contents are thoroughly homogenised. Determine the pH of the resulting solution.
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.40QAP
Related questions
Question
100%

Transcribed Image Text:A 250 cm3 volumetric flask contains exactly 200,0 cm3 of a 0,025 mol.dm3
sulphuric acid solution. Thereafter ten (10) sodium hydroxide pellets, each
of mass 0,1 g are dropped into the flask. After the pellets have dissolved
completely, the flask is topped to the 250 cm3 mark with water and the
contents are thoroughly homogenised. Determine the pH of the resulting
3.
solution.
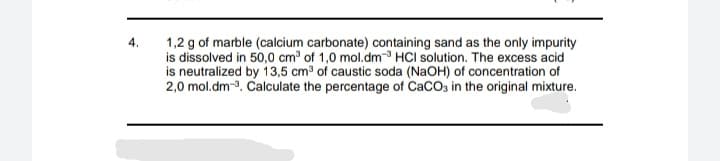
Transcribed Image Text:1,2 g of marble (calcium carbonate) containing sand as the only impurity
is dissolved in 50,0 cm of 1,0 mol.dm- HCI solution. The excess acid
is neutralized by 13,5 cm3 of caustic soda (NaOH) of concentration of
2,0 mol.dm-3. Calculate the percentage of CaCO3 in the original mixture.
4.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you



