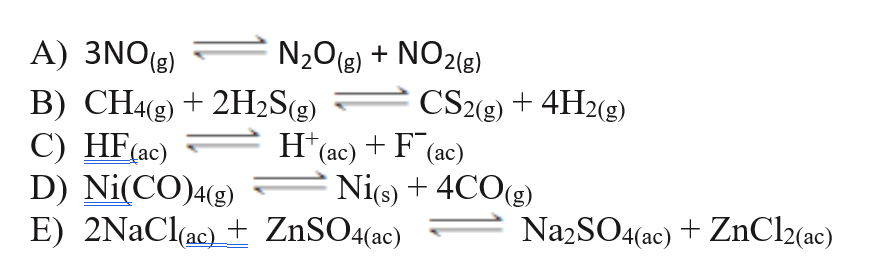Chemistry 9th Edition
ISBN: 9781133611097
Author: Steven S. Zumdahl
Publisher: Steven S. Zumdahl
1 Chemical Foundations 2 Atoms, Molecules, And Ions 3 Stoichiometry 4 Types Of Chemical Reactions And Solution Stoichiometry 5 Gases 6 Thermochemistry 7 Atomic Structure And Periodicity 8 Bonding: General Concepts 9 Covalent Bonding: Orbitals 10 Liquids And Solids 11 Properties Of Solutions 12 Chemical Kinetics 13 Chemical Equilibrium 14 Acids And Bases 15 Acid-base Equilibria 16 Solubility And Complex Ion Equilibria 17 Spontaneity, Entropy, And Free Energy 18 Electrochemistry 19 The Nucleus: A Chemist's View 20 The Representative Elements 21 Transition Metals And Coordination Chemistry 22 Organic And Biological Molecules Chapter14: Acids And Bases
Chapter Questions Section: Chapter Questions
Problem 1RQ: Define each of the following: a. Arrhenius acid b. BronstedLowry acid c. Lewis acid Which of the... Problem 2RQ: Define or illustrate the meaning of the following terms: a. Ka reaction b. Ka equilibrium constant... Problem 3RQ: Define or illustrate the meaning of the following terms: a. amphoteric b. Kw reaction c. Kw... Problem 4RQ: How is acid strength related to the value of Ka? What is the difference between strong acids and... Problem 5RQ: Two strategies are followed when solving for the pH of an acid in water. What is the strategy for... Problem 6RQ: Two strategies are also followed when solving for the pH of a base in water. What is the strategy... Problem 7RQ: Table 13-4 lists the stepwise Ka values for some polyprotic acids. What is the difference between a... Problem 8RQ: For conjugate acidbase pairs, how are Ka and Kb related? Consider the reaction of acetic acid in... Problem 9RQ: What is a salt? List some anions that behave as weak bases in water. List some anions that have no... Problem 10RQ: For oxyacids, how does acid strength depend on a. the strength of the bond to the acidic hydrogen... Problem 1ALQ: Consider two beakers of pure water at different temperatures. How do their pH values compare? Which... Problem 2ALQ: Differentiate between the terms strength and concentration as they apply to acids and bases. When is... Problem 3ALQ: Sketch two graphs: (a) percent dissociation for weak acid HA versus the initial concentration of HA... Problem 4ALQ: Consider a solution prepared by mixing a weak acid HA and HCl. What are the major species? Explain... Problem 5ALQ Problem 6ALQ: Consider two separate aqueous solutions: one of a weak acid HA and one of HCl. Assuming you started... Problem 7ALQ: You are asked to calculate the H+ concentration in a solution of NaOH(aq). Because sodium hydroxide... Problem 8ALQ: Consider a solution prepared by mixing a weak acid HA. HCl, and NaA. Which of the following... Problem 9ALQ: Consider a solution formed by mixing 100.0 mL of 0.10 M HA (Ka = 1.0 106), 100.00 mL of 0.10 M NaA.... Problem 10ALQ: A certain sodium compound is dissolved in water to liberate Na+ ions and a certain negative ion.... Problem 11ALQ: Acids and bases can be thought of as chemical opposites (acids are proton donors, and bases are... Problem 12ALQ: Consider two solutions of the salts NaX(aq) and NaY(aq) at equal concentrations. What would you need... Problem 13ALQ: What is meant by pH? True or false: A strong acid solution always has a lower pH than a weak acid... Problem 14ALQ: Why is the pH of water at 25C equal to 7.00? Problem 15ALQ: Can the pH of a solution be negative? Explain. Problem 16ALQ: Is the conjugate base of a weak acid a strong base? Explain. Explain why Cl does not affect the pH... Problem 17ALQ: Match the following pH values: 1, 2, 5, 6, 6.5, 8, 11, 11, and 13 with the following chemicals (of... Problem 18ALQ: The salt BX, when dissolved in water, produces an acidic solution. Which of the following could be... Problem 19Q: Anions containing hydrogen (for example, HCO3 and H2PO4) usually show amphoteric behavior. Write... Problem 20Q: Which of the following conditions indicate an acidic solution at 25C? a. pH = 3.04 b. [H+] 1.0 107... Problem 21Q: Which of the following conditions indicate a basic solution at 25C? a. pOH = 11.21 b. pH = 9.42 c.... Problem 22Q: Why is H3O+ the strongest acid and OH the strongest base that can exist in significant amounts in... Problem 23Q: How many significant figures are there in the following numbers: 10.78, 6.78, 0.78? If these were pH... Problem 24Q: In terms of orbitals and electron arrangements, what must be present for a molecule or an ion to act... Problem 25Q: Consider the autoionization of liquid ammonia: Label each of the species in the equation as an acid... Problem 26Q: The following are representations of acidbase reactions: a. Label each of the species in both... Problem 27Q: Give three example solutions that fit each of the following descriptions. a. a strong electrolyte... Problem 28Q Problem 29Q Problem 30Q: Which of the following statements is(are) true? Correct the false statements. a. When a base is... Problem 31Q: Consider the following mathematical expressions. a. [H+] = [HA]0 b. [H+] = (Ka [HA]0)1/2 c. [OH] =... Problem 32Q: Consider a 0.10-M H2CO3 solution and a 0.10-M H2SO4 solution. Without doing any detailed... Problem 33Q: Of the hydrogen halides, only HF is a weak acid. Give a possible explanation. Problem 34Q: Explain why the following are done, both of which are related to acidbase chemistry. a. Power plants... Problem 35E: Write balanced equations that describe the following reactions, a. the dissociation of perchloric... Problem 36E: Write the dissociation reaction and the corresponding Ka equilibrium expression for each of the... Problem 37E: For each of the following aqueous reactions, identify the acid, the base, the conjugate base, and... Problem 38E: For each of the following aqueous reactions, identify the acid, the base, the conjugate base, and... Problem 39E: Classify each of the following as a strong acid or a weak acid. Problem 40E: Consider the following illustrations: Which beaker best illustrates what happens when the following... Problem 41E: Use Table 13-2 to order the following from the strongest to the weakest acid. HClO2,H2O,NH4+,HClO4 Problem 42E: Use Table 13-2 to order the following from the strongest to the weakest base. ClO2,H2O,NH3,ClO4 Problem 43E: You may need Table 13-2 to answer the following questions. a. Which is the stronger acid, HCl or... Problem 44E: You may need Table 13-2 to answer the following questions. a. Which is the stronger base, Cl or H2O?... Problem 45E: Calculate the [OH] of each of the following solutions at 25C. Identify each solution as neutral,... Problem 46E: Calculate the [H+] of each of the following solutions at 25C. Identity each solution as neutral,... Problem 47E: Values of Kw as a function of temperature are as follows: Temperature(C) Kw 0 1.14 1015 25 1.00 ... Problem 48E: At 40.C the value of Kw is 2.92 1014. a. Calculate the [H+] and [OH] in pure water at 40.C. b. What... Problem 49E: Calculate the [OH] of each of the following solutions at 25C. Identify each solution as neutral,... Problem 50E: Calculate [H+] and [OH] for each solution at 25C. Identify each solution as neutral, acidic, or... Problem 51E: Fill in the missing information in the following table. Problem 52E: Fill in the missing information in the following table. Problem 53E Problem 54E: The pOH of a sample of baking soda dissolved in water is 5.74 at 25C. Calculate the pH, [H+], and... Problem 55E: What are the major species present in 0.250 M solutions of each of the following acids? Calculate... Problem 56E: A solution is prepared by adding 50.0 mL of 0.050 M HBr to 150.0 mL of 0.10 M HI. Calculate [H+I and... Problem 57E: Calculate the pH of each of the following solutions of a strong acid in water. a. 0.10 M HCl b. 5.0... Problem 58E: Calculate the pH of each of the following solutions containing a strong acid in water. a. 2.0 102 M... Problem 59E: Calculate the concentration of an aqueous HI solution that has pH = 2.50. HI is a strong acid. Problem 60E: Calculate the concentration of an aqueous HBr solution that has pH = 4.25. HBr is a strong acid. Problem 61E: How would you prepare 1600 mL of a pH = 1.50 solution using concentrated (12 M) HCl? Problem 62E: A solution is prepared by adding 50.0 mL concentrated hydrochloric acid and 20.0 mL concentrated... Problem 63E: What are the major species present in 0.250 M solutions of each of the following acids? Calculate... Problem 64E: What are the major species present in 0.250 M solutions of each of the following acids? Calculate... Problem 65E: Calculate the concentration of all species present and the pH of a 0.020-M HF solution. Problem 67E: For propanoic acid (HC3H5O2, Ka = 1.3 105), determine the concentration of all species present, the... Problem 68E: A solution is prepared by dissolving 0.56 g benzoic acid (C6H5CO2H, Ka = 6.4 105) in enough water... Problem 69E: Monochloroacetic acid, HC2H2ClO2, is a skin irritant that is used in chemical peels intended to... Problem 70E: A typical aspirin tablet contains 325 mg acetylsalicylic acid (HC9H7O4). Calculate the pH of a... Problem 72E: A solution is made by adding 50.0 mL of 0.200 M acetic acid (K4 = 1.8 103) to 50.0 mL of 1.00 103... Problem 73E: Calculate the percent dissociation of the acid in each of the following solutions. a. 0.50 M acetic... Problem 74E: Using the Ka values in Table 14.2, calculate the percent dissociation in a 0.20-M solution of each... Problem 75E: A 0.15-M solution of a weak acid is 3.0% dissociated. Calculate Ka. Problem 76E: An acid HX is 25% dissociated in water. If the equilibrium concentration of HX is 0.30 M, calculate... Problem 77E: Trichloroacetic acid (CCl3CO2H) is a corrosive acid that is used to precipitate proteins. The pH of... Problem 78E: The pH of a 0.063-M solution of hypobromous acid (HOBr but usually written HBrO) is 4.95. Calculate... Problem 79E: A solution of formic acid (HCOOH, Ka = 1.8 104) has a pH of 2.70. Calculate the initial... Problem 80E: A typical sample of vinegar has a pH of 3.0. Assuming that vinegar is only an aqueous solution of... Problem 81E: One mole of a weak acid HA was dissolved in 2.0 L of solution. After the system had come to... Problem 82E: You have 100.0 g saccharin, a sugar substitute, and you want to prepare a pH = 5.75 solution. What... Problem 83E: Write the reaction and the corresponding Kb equilibrium expression for each of the following... Problem 84E: Write the reaction and the corresponding Kb equilibrium expression for each of the following... Problem 85E Problem 86E: Use Table 14.3 to help order the following acids from strongest to weakest HNO3,H2O,NH4+,C5H5NH+ Problem 87E: Use Table 14.3 to help answer the following questions. a. Which is the stronger base, ClO4 or... Problem 88E: Use Table 14.3 to help answer the following questions. a. Which is the stronger acid, HClO4 or... Problem 89E: Calculate the pH of the following solutions. a. 0.10 M NaOH b. 1.0 1010 M NaOH c. 2.0 M NaOH Problem 90E: Calculate [OH], pOH, and pH for each of the following. a. 0.00040 M Ca(OH)2 b. a solution containing... Problem 91E: What are the major species present in 0.015 M solutions of each of the following bases? a. KOH b.... Problem 92E: What are the major species present in the following mixtures of bases? a. 0.050 M NaOH and 0.050 M... Problem 93E: What mass of KOH is necessary to prepare 800.0 mL of a solution having a pH = 11.56? Problem 94E: Calculate the concentration of an aqueous Sr(OH)2 that has pH =10.50. Problem 95E: What are the major species present in a 0.150-M NH3 solution? Calculate the [OH] and the pH of this... Problem 96E: For the reaction of hydrazine (N2H4) in water, H2NNH2(aq)+H2O(l)H2NNH3+(aq)+OH(aq) Kb is 3.0 106.... Problem 97E Problem 99E: Calculate the pH of a 0.20-M C2H5NH2 solution (Kb = 5.6 104). Problem 100E: Calculate the pH of a 0.050-M (C2H5)2NH solution(Kb = 1.3 103). Problem 101E: What is the percent ionization in each of the following solutions? a. 0.10 M NH3 b. 0.010 M NH3 c.... Problem 102E: Calculate the percentage of pyridine (C5H5N) that forms pyridinium ion, C5H5NH+, in a 0.10-M aqueous... Problem 103E: The pH of a 0.016-M aqueous solution of p-toluidine (CH3C6H4NH2) is 8.60. Calculate Kb. Problem 104E: Calculate the mass of HONH2 required to dissolve in enough water to make 250.0 mL of solution having... Problem 105E: Write out the stepwise Ka reactions for the diprotic acid H2SO3. Problem 106E: Write out the stepwise Ka reactions for citric acid (H3C6H5O7), a triprotic acid. Problem 107E: A typical vitamin C tablet (containing pure ascorbic acid, H2C6H6O6) weighs 500. mg. One vitamin C... Problem 108E: Arsenic acid (H3AsO4) is a triprotic acid with Ka1 = 5.5 103, Ka2 = 1.7 107, and Ka3 = 5.1 1012.... Problem 109E: Calculate the pH and [S2] in a 0.10-M H2S solution. Assume Ka1 = 1.0 107; Ka2 = 1.0 1019. Problem 110E: Calculate [CO32] in a 0.010-M solution of CO2 in water (usually written as H2CO3). If all the CO32... Problem 111E: Calculate the pH of a 2.0-M H2SO4 solution. Problem 112E: Calculate the pH of a 5.0 103-M solution of H2SO4. Problem 113E: Arrange the following 0.10 M solutions in order of most acidic to most basic. KOH,KNO3,KCN,NH4Cl,HCl Problem 114E: Arrange the following 0.10 M solutions in order from most acidic to most basic. See Appendix 5 for... Problem 115E: Given that the Ka value for acetic acid is 1.8 105 and the Ka value for hypochlorous acid is 3.5 ... Problem 116E: The Kb values for ammonia and methylamine are 1.8 105 and 4.4 104, respectively. Which is the... Problem 117E: Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2 Problem 118E: Calculate the concentrations of all species present in a 0.25-M solution of ethylammonium chloride... Problem 119E: Calculate the pH of each of the following solutions. a. 0.10 M CH3NH3Cl b. 0.050 M NaCN Problem 120E: Calculate the pH of each of the following solutions. a. 0.12 M KNO2 b. 0.45 M NaOCl c. 0.40 M... Problem 121E: Sodium azide (NaN3) is sometimes added to water to kill bacteria. Calculate the concentration of all... Problem 122E: Papaverine hydrochloride (abbreviated papH+Cl; molar mass = 378.85 g/mol) is a drug that belongs to... Problem 123E: An unknown salt is either NaCN, NaC2H3O2, NaF, NaCl, or NaOCl. When 0.100 mole of the salt is... Problem 124E: Consider a solution of an unknown salt having the general formula BHCl, where B is one of the weak... Problem 125E: A 0.050-M solution of the salt NaB has a pH of 9.00. Calculate the pH of a 0.010-M solution of HB. Problem 126E: A 0.20-M sodium chlorobenzoate (NaC7H4ClO2) solution has a pH of 8.65. Calculate the pH of a 0.20-M... Problem 127E Problem 128E Problem 129E: Are solutions of the following salts acidic, basic, or neutral? For those that are not neutral,... Problem 130E: Are solutions of the following salts acidic, basic, or neutral? For those that are not neutral,... Problem 131E: Place the species in each of the following groups in order of increasing acid strength. Explain the... Problem 132E: Place the species in each of the following groups in order of increasing base strength. Give your... Problem 133E: Place the species in each of the following groups in order of increasing acid strength. a. H2O, H2S,... Problem 134E: Using your results from Exercise 133, place the species in each of the following groups in order of... Problem 135E: Will the following oxides give acidic, basic, or neutral solutions when dissolved in water? Write... Problem 136E: Will the following oxides give acidic, basic, or neutral solutions when dissolved in water? Write... Problem 137E: Identify the Lewis acid and the Lewis base in each of the following reactions. a.... Problem 138E: Identify the Lewis acid and the Lewis base in each of the following reactions. a.... Problem 139E: Aluminum hydroxide is an amphoteric substance. It can act as either a Brnsted-Lowry base or a Lewis... Problem 140E: Zinc hydroxide is an amphoteric substance. Write equations that describe Zn(OH)2 acting as a... Problem 141E: Would you expect Fe3+ or Fe2+ to be the stronger Lewis acid? Explain. Problem 142E Problem 143AE: A 10.0-mL sample of an HCl solution has a pH of 2.000. What volume of water must be added to change... Problem 144AE: Which of the following represent conjugate acidbase pairs? For those pairs that are not conjugates,... Problem 145AE: A solution is tested for pH and conductivity as pictured below: The solution contains one of the... Problem 146AE: The pH of human blood is steady at a value of approximately 7.4 owing to the following equilibrium... Problem 147AE: Hemoglobin (abbreviated Hb) is a protein that is responsible for the transport of oxygen in the... Problem 148AE: A 0.25-g sample of lime (CaO) is dissolved in enough water to make 1500 mL of solution. Calculate... Problem 149AE: At 25C, a saturated solution of benzoic acid (Ka = 6.4 105) has a pH of 2.80. Calculate the water... Problem 150AE: Calculate the pH of an aqueous solution containing 1.0 102 M HCl, 1.0 102 M H2SO4.and 1.0 102... Problem 151AE: Acrylic acid (CH29CHCO2H) is a precursor for many important plastics. Ka for acrylic acid is 5.6 ... Problem 152AE: Classify each of the following as a strong acid, weak acid, strong base, or weak base in aqueous... Problem 153AE: The following illustration displays the relative number of species when an acid, HA, is added to... Problem 154AE: Quinine (C20H24N2O2) is the most important alkaloid derived from cinchona bark. It is used as an... Problem 155AE: Codeine (C18H21NO3) is a derivative of morphine that is used as an analgesic, narcotic, or... Problem 156AE: A codeine-containing cough syrup lists codeine sulfate as a major ingredient instead of codeine. The... Problem 157AE Problem 158AE: Rank the following 0.10 M solutions in order of increasing pH. a. HI, HF, NaF, NaI b. NH4Br, HBr,... Problem 159AE: Is an aqueous solution of NaHSO4 acidic, basic, or neutral? What reaction occurs with water?... Problem 160AE: Calculate the value for the equilibrium constant for each of the following aqueous reactions. a.... Problem 161AE Problem 162CWP: For solutions of the same concentration, as acid strength increases, indicate what happens to each... Problem 163CWP Problem 164CWP: Consider a 0.60-M solution of HC3H5O3, lactic acid (Ka = 1.4 104). a. Which of the following are... Problem 165CWP: Consider a 0.67-M solution of C2H5NH2 (Kb = 5.6 104). a. Which of the following are major species... Problem 166CWP: Rank the following 0.10 M solutions in order of increasing pH. a. NH3 b. KOH c. HC2H3O2 d. KCl e.... Problem 167CWP: Consider 0.25 M solutions of the following salts: NaCl, RbOCl, KI, Ba(ClO4)2, and NH4NO3. For each... Problem 168CWP: Calculate the pH of the following solutions: a. 1.2 M CaBr2 b. 0.84 M C6H5NH3NO3 (Kb for C6H5NH2 =... Problem 169CWP: Consider 0.10 M solutions of the following compound: AlCl3, NaCN, KOH, CsClO4, and NaF. Place these... Problem 170CP: The pH of 1.0 108 M hydrochloric acid is not 8.00. The correct pH can be calculated by considering... Problem 171CP: Calculate the pH of a 1.0 107-M solution of NaOH in water. Problem 172CP: Calculate [OH] in a 3.0 107-M solution of Ca(OH)2. Problem 173CP: Consider 50.0 mL of a solution of weak acid HA (Ka = 1.00 106), which has a pH of 4.000. What... Problem 174CP Problem 175CP: Calculate the pH of a 0.200-M solution of C5H5NHF. Hint: C5H5NHF is a salt composed of C5H5NH+ and F... Problem 176CP: Determine the pH of a 0.50-M solution of NH4OCl. (See Exercise 175.) Problem 177CP: Calculate [OH] in a solution obtained by adding 0.0100 mol solid NaOH to 1.00 L of 15.0 M NH3. Problem 178CP: What mass of NaOH(s) must be added to 1.0 L of 0.050 M NH3 to ensure that the percent ionization of... Problem 179CP: Consider 1000. mL of a 1.00 104-M solution of a certain acid HA that has a Ka value equal to 1.00 ... Problem 180CP: Calculate the mass of sodium hydroxide that must be added to 1.00 L of 1.00-M HC2H3O2 to double the... Problem 181CP: Consider the species PO43, HPO42, and H2PO4. Each ion can act as a base in water. Determine the Kb... Problem 182CP: Calculate the pH of a 0.10-M solution of sodium phosphate. (See Exercise 181.) Problem 183CP: Will 0.10 M solutions of the following salts be acidic, basic, or neutral? See Appendix 5 for Ka... Problem 184CP: a. The principal equilibrium in a solution of NaHCO3 is HCO3(aq)+HCO3(aq)H2CO3(aq)+CO32(aq)... Problem 185CP: A 0.100-g sample of the weak acid HA (molar mass = 100.0 g/mol) is dissolved in 500.0 g water. The... Problem 186CP: A sample containing 0.0500 mole of Fe2(SO4)3 is dissolved in enough water to make 1.00 L of... Problem 187IP: A 2.14 g sample of sodium hypoiodite is dissolved in water to make 1.25 L of solution. The solution... Problem 188IP: Isocyanic acid (HNCO) can be prepared by heating sodium cyanate in the presence of solid oxalic acid... Problem 189IP: A certain acid, HA, has a vapor density of 5.11 g/L when in the gas phase at a temperature of 25C... Problem 190MP: An aqueous solution contains a mixture of 0.0500 M HCOOH (Ka = 1.77 104) and 0.150 M CH3CH2COOH (Ka... Problem 191MP: For the following, mix equal volumes of one solution from Group I with one solution from Group II to... Problem 52E: Fill in the missing information in the following table.
Related questions
Concept explainers
7. In the reactions below, mark the one that does not correspond to a homogeneous equilibrium :
Transcribed Image Text: A) 3NO(g)
N2O(g) +
NO2(8)
CS2(g) + 4H2(g)
B) CH4(g) + 2H2S(g)
C) HF(ac)
D) Ni(CO)4(g)
E) 2NACI(ac) + ZnSO4(ac)
H*(ac) + F (ac)
Ni(s) + 4CO(g)
NazSO4(ac) + ZnCl2(ac)
Definition Definition State in which all the components involved in a reaction occur in the same state. A homogeneous equilibrium is observed in solutions where all the components are in a dissolved state, whereas for gaseous equilibrium all the components are in a gaseous state.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images