How many rings are in X? How many double bonds are in X? Show your work. 8. continued Compound X, C37H54BT4, reacts with excess H2/Pd to give a C37H62Br4 compound.
How many rings are in X? How many double bonds are in X? Show your work. 8. continued Compound X, C37H54BT4, reacts with excess H2/Pd to give a C37H62Br4 compound.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter21: Nas: Nucleophilic Aromatic Substitution
Section: Chapter Questions
Problem 7E
Related questions
Question
Please help me answer these questions thank you so much!!
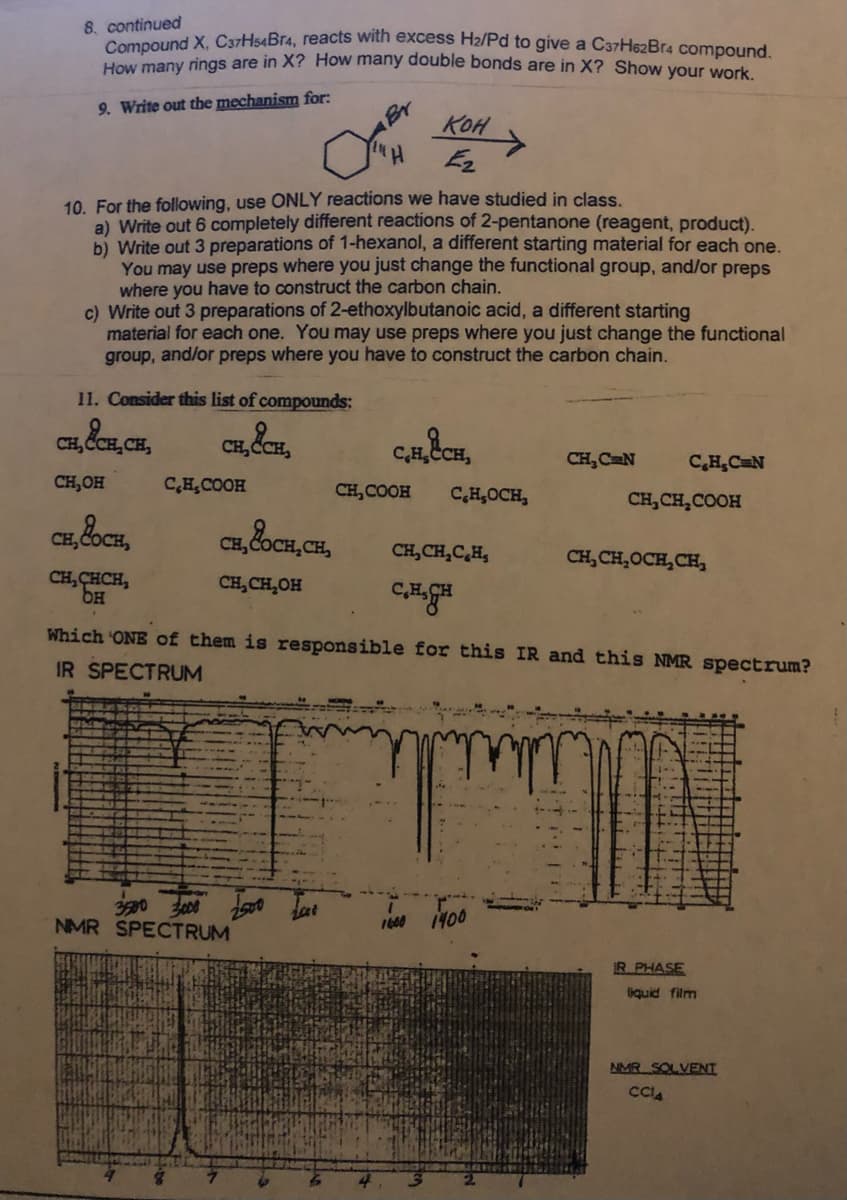
Transcribed Image Text:8. continued
Compound X, C37H54Br4, reacts with excess H2/Pd to give a C37He2Br4 compound
How many rings are in X? How many double bonds are in X? Show your work
Br
KOH
9. Write out the mechanism for:
Hi
10. For the following, use ONLY reactions we have studied in class.
a) Write out 6 completely different reactions of 2-pentanone (reagent, product).
b) Write out 3 preparations of 1-hexanol, a different starting material for each one.
You may use preps where you just change the functional group, and/or preps
where you have to construct the carbon chain.
c) Write out 3 preparations of 2-ethoxylbutanoic acid, a different starting
material for each one. You may use preps where you just change the functional
group, and/or preps where you have to construct the carbon chain.
11. Consider this list of compounds:
CH, ČCE, CH,
CH C
C,HCH
CH, C N
C,H,CN
CH,OH
C,HCOOH
CH, COOH
C,H,OCH,
CH, CH,COOH
CH,C
CH, &oCH, CH,
CH,CH,C,H,
CH, CH,OCH,CH,
CH, CHCH,
CH,CH,OH
Which ONE of them is responsible for this IR and this NMR spectrum?
IR SPECTRUM
NMR SPECTRUM
1600 1400
R.PHASE
liquid film
NMR SOLVENT
CCA
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning