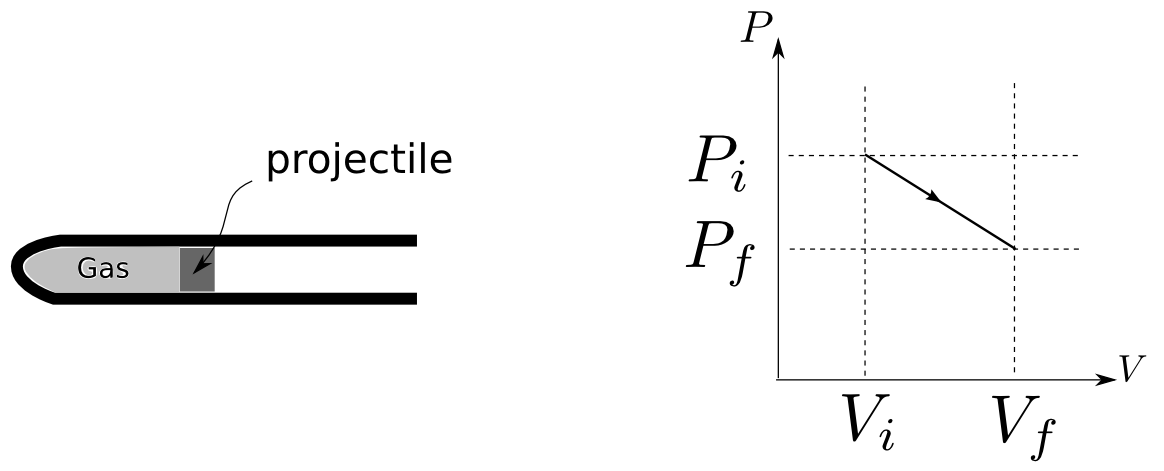
A 40.0g projectile is launched by the expansion of hot gases as shown. The cross sectional area of the launch tube is 1.0cm^2 and the length the projectile travels down the tube is 32cm. As the gas expands, the pressure and volume varies as shown. The initial pressure and volume are 11e5 Pa, and 8.0cm^3, and the final pressure and volume is 1.0e5Pa and 40.0cm^3. Friction with the launch tube is negligible. a) If the projectile is fired into vacuum, what is the speed of the projectile when it leaves the tube? b) If the projectile is launched into a room where the air pressure is 1.0e5Pa, how much energy is spent pushing the air out of the way as the projectile moves down the barrel? (Hint: What is the work exerted on the projectile by the air in the room? Also, think about which thermodynamic variable(s), describing the air in the room, you can approximate as constant.)
A 40.0g projectile is launched by the expansion of hot gases as shown.
The cross sectional area of the launch tube is 1.0cm^2 and the length the projectile travels down the tube is 32cm. As the gas expands, the pressure and volume varies as shown. The initial pressure and volume are 11e5 Pa, and 8.0cm^3, and the final pressure and volume is 1.0e5Pa and 40.0cm^3. Friction with the launch tube is negligible.
a) If the projectile is fired into vacuum, what is the speed of the projectile when it leaves the tube?
b) If the projectile is launched into a room where the air pressure is 1.0e5Pa, how much energy is spent pushing the air out of the way as the projectile moves down the barrel? (Hint: What is the work exerted on the projectile by the air in the room? Also, think about which
Answer both parts
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images









