a) 453 mg to g b) 6.0 x 10-9: m to pm c) 2.5 x 10-2 mm to um d) 3.85 x 10° g to kg e) 0.0065 mm to nm f) 3.05 x 10-ls to ms a) 2.75 kg/m to g/L b) 8.64 x 10-3 nm? to m? c) 2.082 cm-1 to m-1
a) 453 mg to g b) 6.0 x 10-9: m to pm c) 2.5 x 10-2 mm to um d) 3.85 x 10° g to kg e) 0.0065 mm to nm f) 3.05 x 10-ls to ms a) 2.75 kg/m to g/L b) 8.64 x 10-3 nm? to m? c) 2.082 cm-1 to m-1
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter3: Measurement And Chemical Calculations
Section: Chapter Questions
Problem 44E: a 15.3 kg to g and mg, b 80.5 g to kg and mg, c 58.5 mg to kg and g
Related questions
Question
100%
Perform each of the unit conversions shown below:
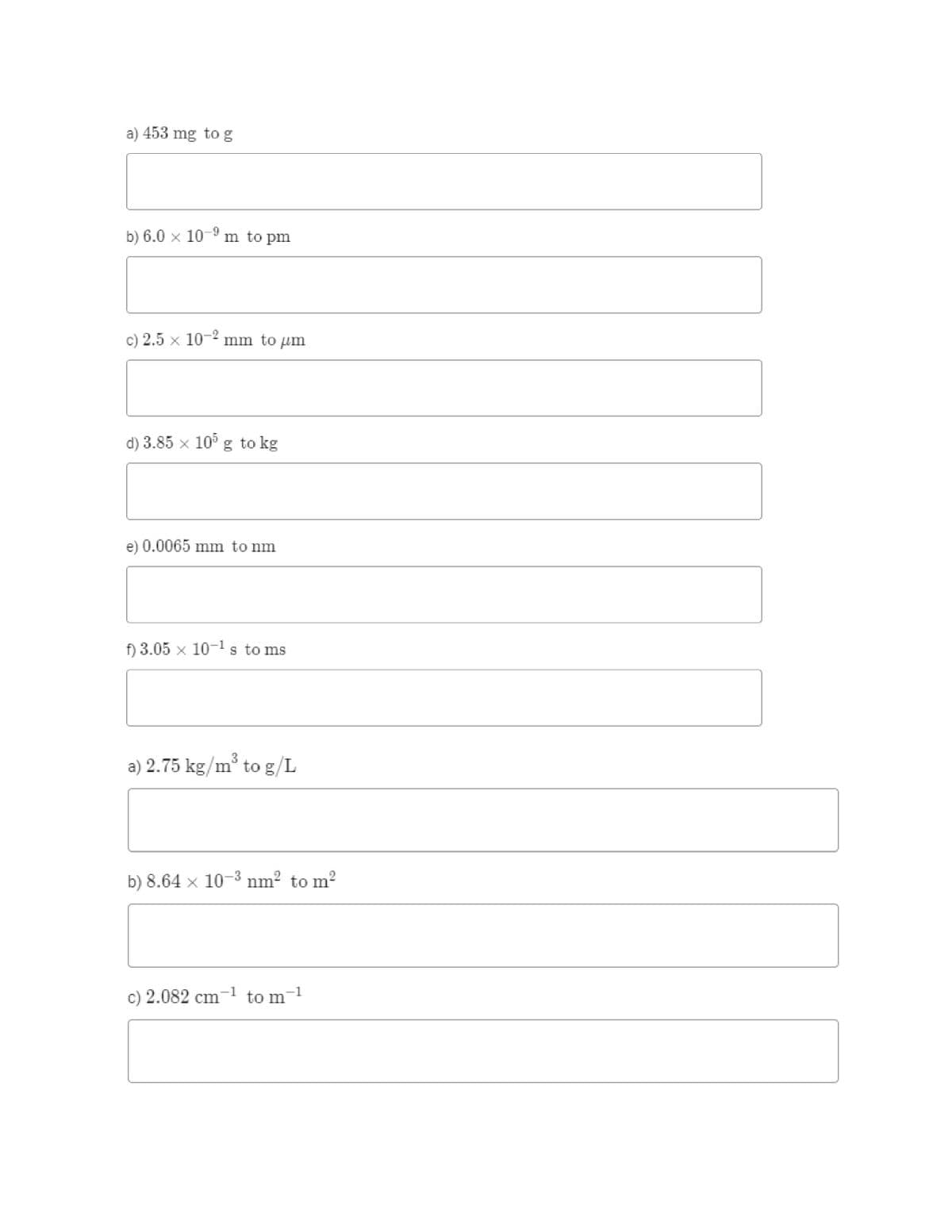
Transcribed Image Text:a) 453 mg to g
b) 6.0 x 10-9 m to pm
c) 2.5 x 10-" mm to um
d) 3.85 x 10° g to kg
e) 0.0065 mm to nm
f) 3.05 x 10-ls to ms
3
a) 2.75 kg/m° to g/L
b) 8.64 x 10-3 nm? to m?
c) 2.082 cm
-1
to m
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning