Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter12: Chemical Bonding
Section: Chapter Questions
Problem 17CR: Schrodinger and de Broglie suggested a ‘Wave—particle duality" for small particles—that is, if...
Related questions
Question
Transcribed Image Text:101 Chem101
b Chemistry Question | bartleby x Q Fichas de aprendizaje Noble Ga X +
app.101edu.co
M
Apps
G
M Gmail
YouTube
Maps
a AMAZON
Translate
Gflights
Case Status Onlin...
b Homework Help a...
C Get Homework He... > KATAPULK CUBA
23 Agencia Supermar..
Reading List
Question 7 of 22
Submit
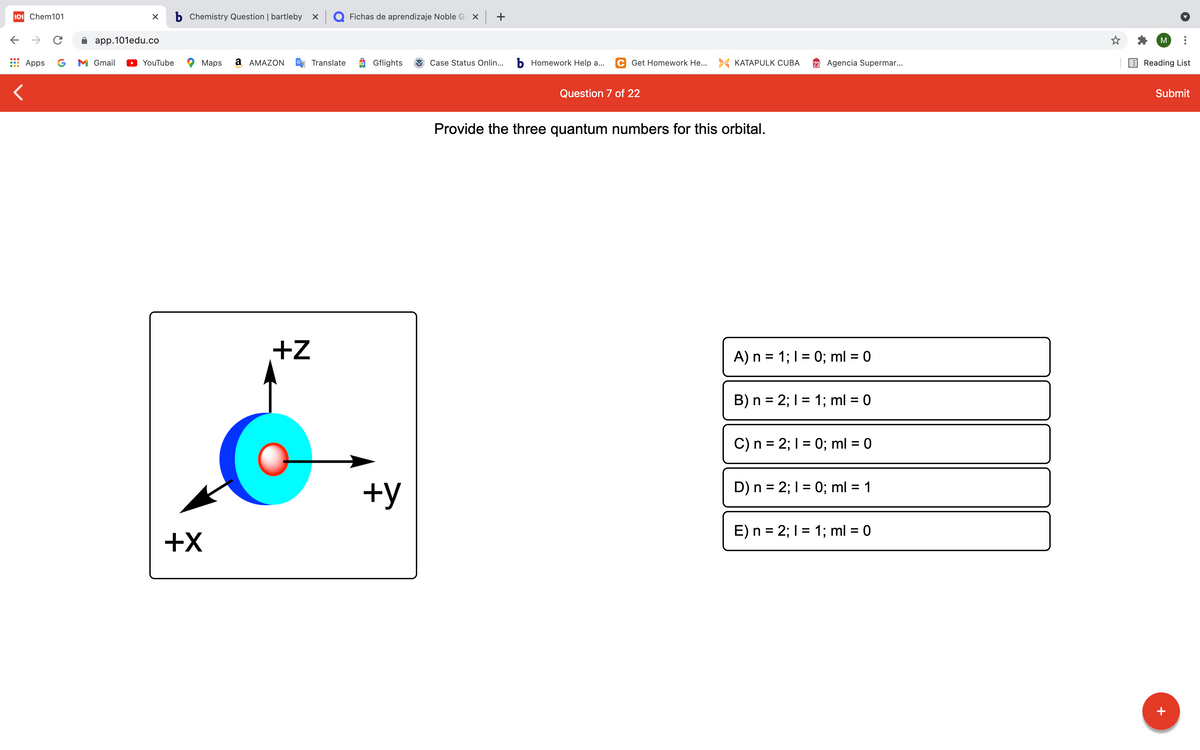
Provide the three quantum numbers for this orbital.
+Z
A) n = 1; 1 = 0; ml = 0
B) n = 2; 1 = 1; ml = 0
%D
C) n = 2; I = 0; ml = 0
D) n = 2; I = 0; ml = 1
+y
E) n = 2; I = 1; ml = 0
+X
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co