A About the Program | College of X e Minors for Nol A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-liJOkWynm4w-aQ-rw-zRhgRnayfmbBs O STATES OF MATTER Using the combined gas law 3/5 For many purposes we can treat nitrogen (N,) as an ideal gas at temperatures above its boiling point of -196. °C. Suppose the temperature of a sample of nitrogen gas is lowered from -19.0 °C to -57.0 °C, and at the same time the pressure is increased by 5.0%. O increase Does the volume of the sample increase, decrease, or stay the same? O decrease O stays the same If you said the volume increases or decreases, calculate the percentage change in| % the volume. Round your answer to the nearest percent. Explanation Check O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Pri Aa W 30
A About the Program | College of X e Minors for Nol A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-liJOkWynm4w-aQ-rw-zRhgRnayfmbBs O STATES OF MATTER Using the combined gas law 3/5 For many purposes we can treat nitrogen (N,) as an ideal gas at temperatures above its boiling point of -196. °C. Suppose the temperature of a sample of nitrogen gas is lowered from -19.0 °C to -57.0 °C, and at the same time the pressure is increased by 5.0%. O increase Does the volume of the sample increase, decrease, or stay the same? O decrease O stays the same If you said the volume increases or decreases, calculate the percentage change in| % the volume. Round your answer to the nearest percent. Explanation Check O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Pri Aa W 30
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.127QP: A 1.000-g sample of an unknown gas at 0C gives the following data: P(atm) V (L) 0.2500 3.1908 0.5000...
Related questions
Question
100%
Please see image
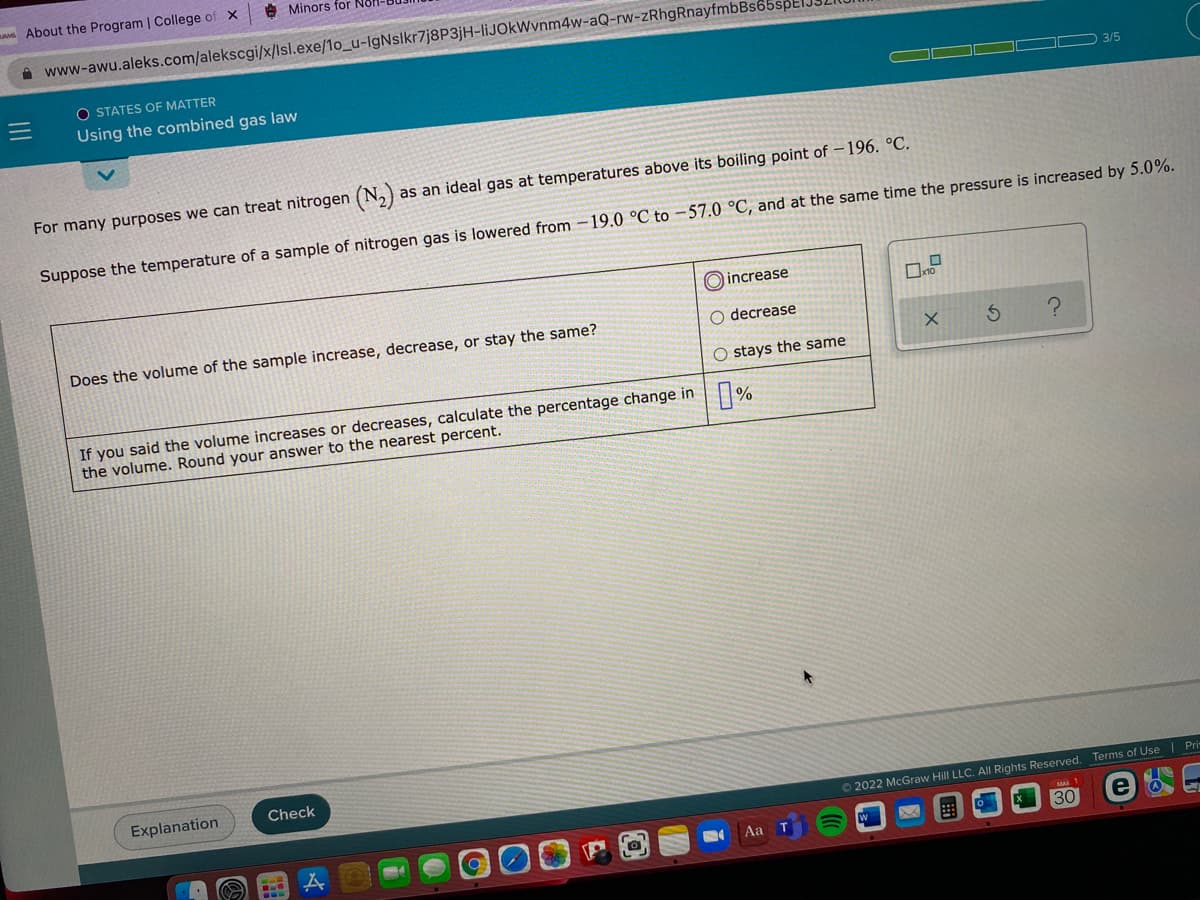
Transcribed Image Text:A About the Program | College of X
A Minors for Nol
A www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-liJOkWynm4w-aQ-rw-zRhgRnayfmbBs
O STATES OF MATTER
Using the combined gas law
3/5
For many purposes we can treat nitrogen (N,)
as an ideal gas at temperatures above its boiling point of -196. °C.
Suppose the temperature of a sample of nitrogen gas is lowered from -19.0 °C to -57.0 °C, and at the same time the pressure is increased by 5.0%.
O increase
Does the volume of the sample increase, decrease, or stay the same?
O decrease
O stays the same
If you said the volume increases or decreases, calculate the percentage change in %
the volume. Round your answer to the nearest percent.
Explanation
Check
O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Pri
Aa
W
30
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning