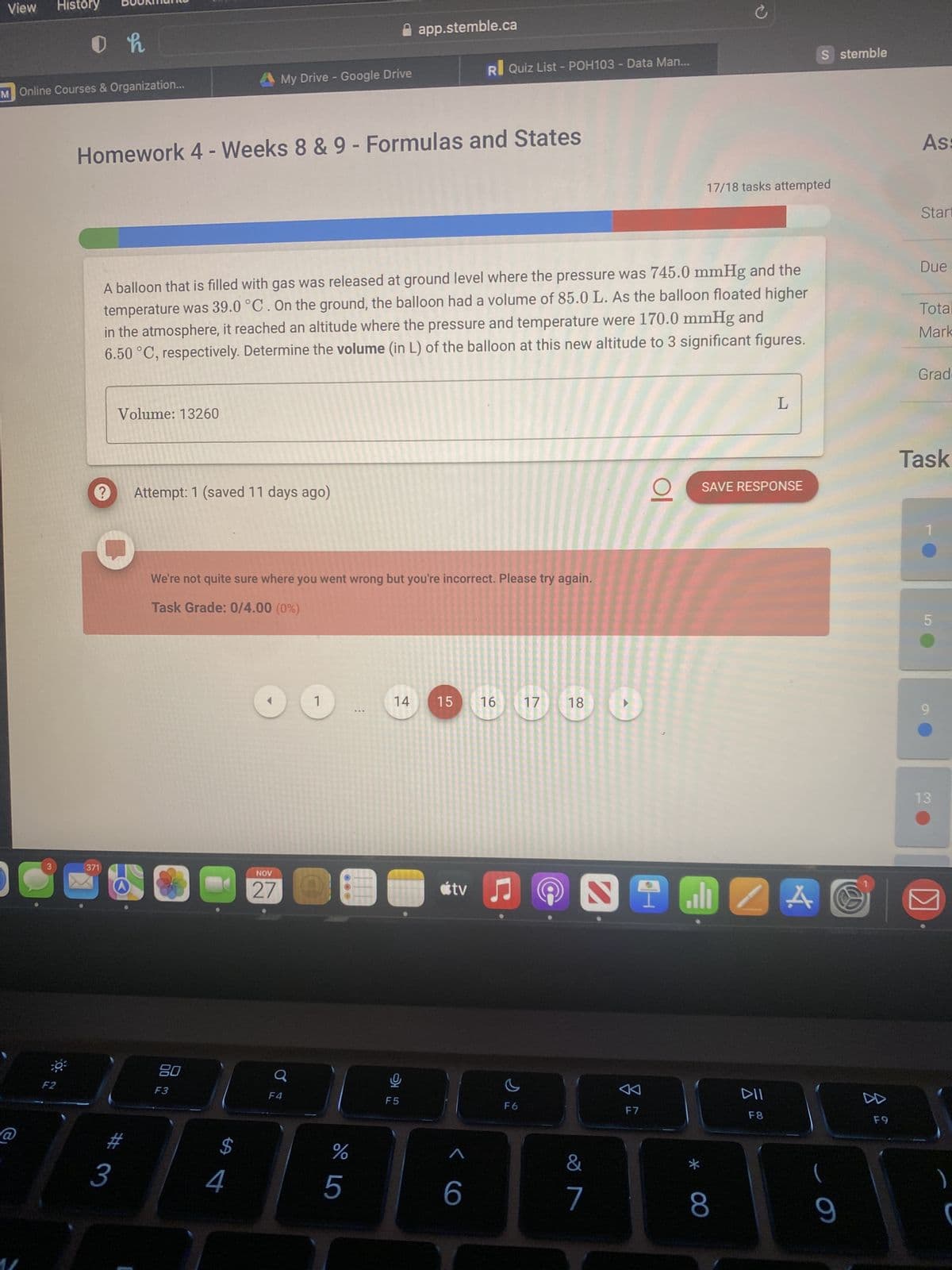
A balloon that is filled with gas was released at ground level where the pressure was 745.0 mmHg an temperature was 39.0 °C. On the ground, the balloon had a volume of 85.0 L. As the balloon floated higher in the atmosphere, it reached an altitude where the pressure and temperature were 170.0 mmHg and 6.50 °C, respectively. Determine the volume (in L) of the balloon at this new altitude to 3 significant figures.
A balloon that is filled with gas was released at ground level where the pressure was 745.0 mmHg an temperature was 39.0 °C. On the ground, the balloon had a volume of 85.0 L. As the balloon floated higher in the atmosphere, it reached an altitude where the pressure and temperature were 170.0 mmHg and 6.50 °C, respectively. Determine the volume (in L) of the balloon at this new altitude to 3 significant figures.
Chapter5: Gases
Section: Chapter Questions
Problem 143CWP: A certain flexible weather balloon contains helium gas at a volume of 855 L. Initially, the balloon...
Related questions
Question
Transcribed Image Text:View
@
History
M Online Courses & Organization...
:9
F2
0 h
371
Homework 4 - Weeks 8 & 9 - Formulas and States
Volume: 13260
#
3
A balloon that is filled with gas was released at ground level where the pressure was 745.0 mmHg and the
temperature was 39.0 °C. On the ground, the balloon had a volume of 85.0 L. As the balloon floated higher
in the atmosphere, it reached an altitude where the pressure and temperature were 170.0 mmHg and
6.50 °C, respectively. Determine the volume (in L) of the balloon at this new altitude to 3 significant figures.
Attempt: 1 (saved 11 days ago)
My Drive - Google Drive
80
F3
We're not quite sure where you went wrong but you're incorrect. Please try again.
Task Grade: 0/4.00 (0%)
4
NOV
27
F4
1
%
app.stemble.ca
от оо
5
R Quiz List - POH103 - Data Man...
F5
14 15 16 17 18
tv♫♬
ㄱ
6
F6
&
7
2
F7
ol
C
*
17/18 tasks attempted
alı 2
SAVE RESPONSE
8
L
DII
F8
S stemble
4.
(
9
F9
Ass
Start
Due
Total
Mark
Grad
Task
1
5
9
13
D
(
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning