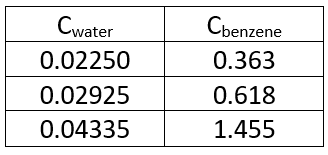
(a) Based on the tabulated data, prove that the distribution of benzoic acid in water and and benzene follows C2water/Cbenzene=K. Cite the possible relations and assumptions. (b) Determine the moles of benzoic acid which may be extracted from 150 cc of a 0.3 molar aqueous solution 15 cc of benzene at 20oC by (1) single extraction and by (2) five extractions.
(a) Based on the tabulated data, prove that the distribution of benzoic acid in water and and benzene follows C2water/Cbenzene=K. Cite the possible relations and assumptions. (b) Determine the moles of benzoic acid which may be extracted from 150 cc of a 0.3 molar aqueous solution 15 cc of benzene at 20oC by (1) single extraction and by (2) five extractions.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 16QAP: Computers are not supposed to be in very warm rooms. The highest temperature tolerated for maximum...
Related questions
Question
(a) Based on the tabulated data, prove that the distribution of benzoic acid in water and and benzene follows C2water/Cbenzene=K. Cite the possible relations and assumptions.
(b) Determine the moles of benzoic acid which may be extracted from 150 cc of a 0.3 molar aqueous solution 15 cc of benzene at 20oC by (1) single extraction and by (2) five extractions.
Transcribed Image Text:Cwater
Cbenzene
0.02250
0.363
0.02925
0.618
0.04335
1.455
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning