(a) Benjamin List and David W.C. MacMillan have been awarded 2021 Nobel Prize in Chemistry, for their development of a new type of catalysis. Amino acid proline, acting as the catalyst, has successfully catalyzed an aldol reaction with the formation of one mirror image of the product much more than the other one. Below is the aldol product: он 4-hydroxy-3-methylpent-2-one The compound has two chiral carbons. Show two of the chiral carbons using asterisk signs and draw one of the chiral carbons in enantiomeric forms. Apply (R) and (S) configurations for the enantiomers based on the Cahn-Ingold-Prelog system. (b) Give the product and the type of mechanism of the reaction of hydrosulfide (HS) with (R)- 2-iodopentane. Give the stereochemistry resulting from the product.
(a) Benjamin List and David W.C. MacMillan have been awarded 2021 Nobel Prize in Chemistry, for their development of a new type of catalysis. Amino acid proline, acting as the catalyst, has successfully catalyzed an aldol reaction with the formation of one mirror image of the product much more than the other one. Below is the aldol product: он 4-hydroxy-3-methylpent-2-one The compound has two chiral carbons. Show two of the chiral carbons using asterisk signs and draw one of the chiral carbons in enantiomeric forms. Apply (R) and (S) configurations for the enantiomers based on the Cahn-Ingold-Prelog system. (b) Give the product and the type of mechanism of the reaction of hydrosulfide (HS) with (R)- 2-iodopentane. Give the stereochemistry resulting from the product.
Chapter19: Aldehydes And Ketones: Nucleophilic Addition Reactions
Section19.SE: Something Extra
Problem 50MP
Related questions
Question
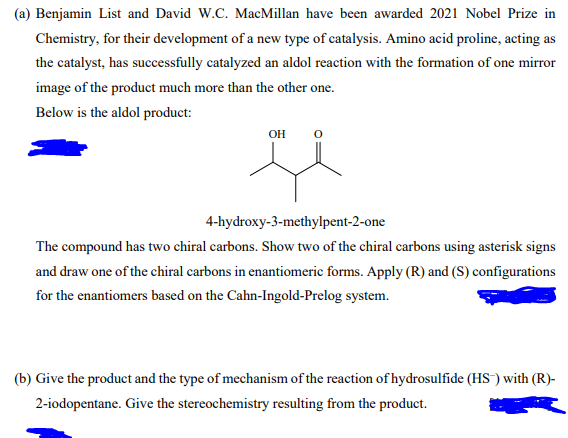
Transcribed Image Text:(a) Benjamin List and David W.C. MacMillan have been awarded 2021 Nobel Prize in
Chemistry, for their development of a new type of catalysis. Amino acid proline, acting as
the catalyst, has successfully catalyzed an aldol reaction with the formation of one mirror
image of the product much more than the other one.
Below is the aldol product:
OH
4-hydroxy-3-methylpent-2-one
The compound has two chiral carbons. Show two of the chiral carbons using asterisk signs
and draw one of the chiral carbons in enantiomeric forms. Apply (R) and (S) configurations
for the enantiomers based on the Cahn-Ingold-Prelog system.
(b) Give the product and the type of mechanism of the reaction of hydrosulfide (HS) with (R)-
2-iodopentane. Give the stereochemistry resulting from the product.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

