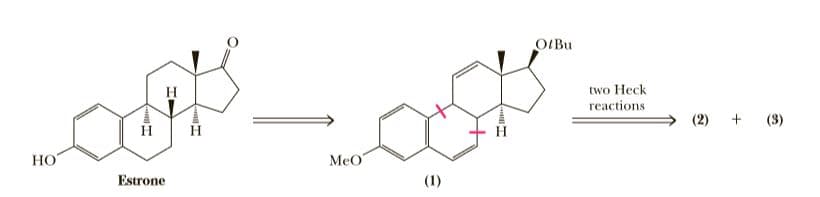
Over the past several decades, chemists have developed a number of synthetic methodologies for the synthesis of steroid hormones. One of these, developed by Lutz Tietze at the Institut für Organische Chemie der Georg-August-Universität, Göttingen, Germany, used a double Heck reaction to create ring B of the steroid nucleus. As shown in the following retrosynthetic analysis, a key intermediate in his synthesis is compound (1). Two Heck reaction disconnects of this intermediate give compounds (2) and (3). Compound (2) contains the
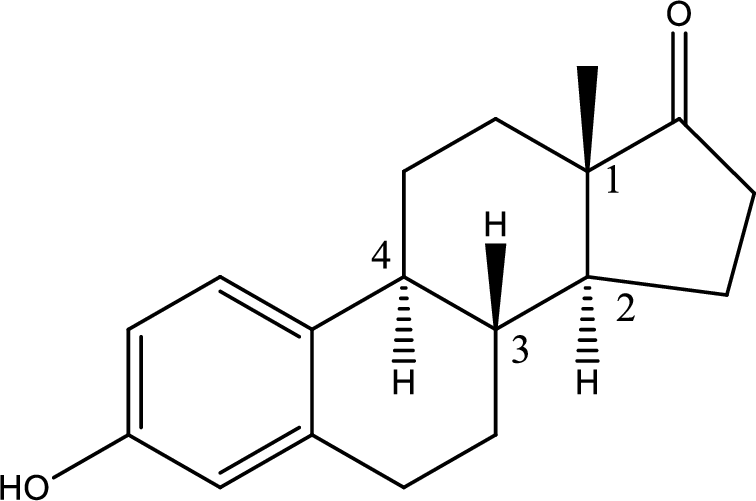
Q.How many chiral centers are present in estrone?
There are four chiral centres in the estrone that are marked in the structure below,
Step by step
Solved in 2 steps with 1 images




