Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter13: The Chemistry Of Solutes And Solutions
Section: Chapter Questions
Problem 42QRT
Related questions
Question
To my understanding that you are supposed to answer 3 questions per asking . Write clear and mine form as questions thank you
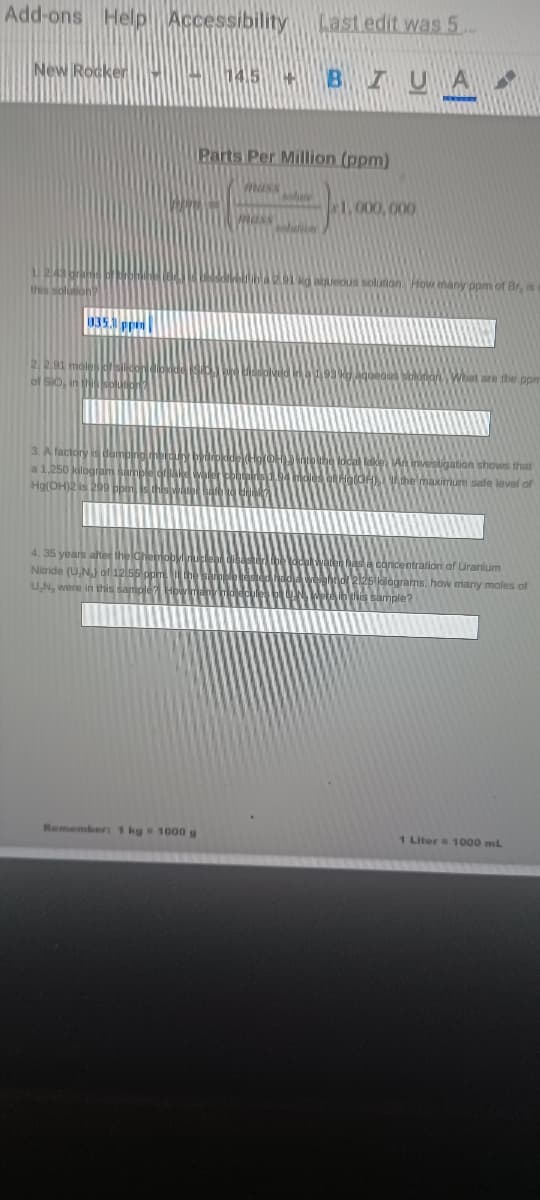
Transcribed Image Text:Add-ons Help Accessibility Last edit was 5.
New RockerHO
H45 + B I UA
Parts Per Million (ppm)
1.000, 000
bueous solution How many ppm of Br, is
135.1 ppin
2201
of Sio,
HA49 kaqueaun solunon What are the ppm
3. A factory is
a 1.250 kibg T AN
Ha(DH)2 is
ligation shows that
he maximum safe level of
4. 35 years alter the o
Nitride (UNj of12 ssp
cancentration af Uranium
25 klágrams, how many moles of
his sample?
UN, were in this samole
Remember: 1 kg 1000g
1 Litera 1000 mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
