A Br substituent is an ortho/para director, so the halogenation of bromobenzene predominantly yields the ortho and para products, as shown in the following bromination and chlorination reactions: Br Br Br Br Br Br Br .CI Br2 Cl2 FeBr3 FeCl3 Br CI 13% 85% 42% 53% Explain why bromination yields more of the para product than chlorination.
A Br substituent is an ortho/para director, so the halogenation of bromobenzene predominantly yields the ortho and para products, as shown in the following bromination and chlorination reactions: Br Br Br Br Br Br Br .CI Br2 Cl2 FeBr3 FeCl3 Br CI 13% 85% 42% 53% Explain why bromination yields more of the para product than chlorination.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter20: Dienes, Conjugated Systems, And Pericyclic Reactions
Section: Chapter Questions
Problem 20.49P
Related questions
Question
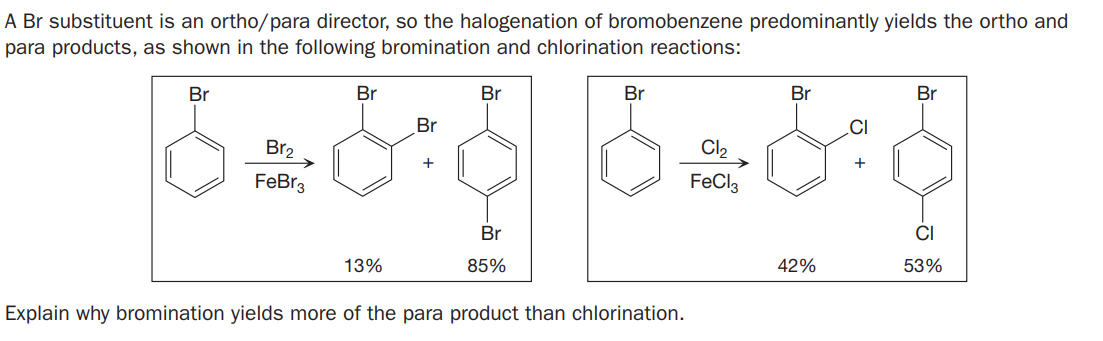
Transcribed Image Text:A Br substituent is an ortho/para director, so the halogenation of bromobenzene predominantly yields the ortho and
para products, as shown in the following bromination and chlorination reactions:
Br
Br
Br
Br
Br
Br
Br
.CI
Br2
Cl2
FeBr3
FeCl3
Br
CI
13%
85%
42%
53%
Explain why bromination yields more of the para product than chlorination.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning