A buffer is prepared by adding 9.00 g of ammonium chloride (NH4C1) to 250 mL of 1.00 M NH3 solution. Write the complete ionic equation for the reaction that occurs when a few drops of potassium hydroxide solution are added to the buffer. Only consider the species reacting when this addition is made. Express your answer as a chemical equation. Identify all of the phases in your answer. ΑΣφ a a ха х Хь 1L
A buffer is prepared by adding 9.00 g of ammonium chloride (NH4C1) to 250 mL of 1.00 M NH3 solution. Write the complete ionic equation for the reaction that occurs when a few drops of potassium hydroxide solution are added to the buffer. Only consider the species reacting when this addition is made. Express your answer as a chemical equation. Identify all of the phases in your answer. ΑΣφ a a ха х Хь 1L
Chapter7: Neutralization Titrations And Graphical Representations
Section: Chapter Questions
Problem 8P
Related questions
Question
Can someone help me answer this problem. I keep getting it wrong.
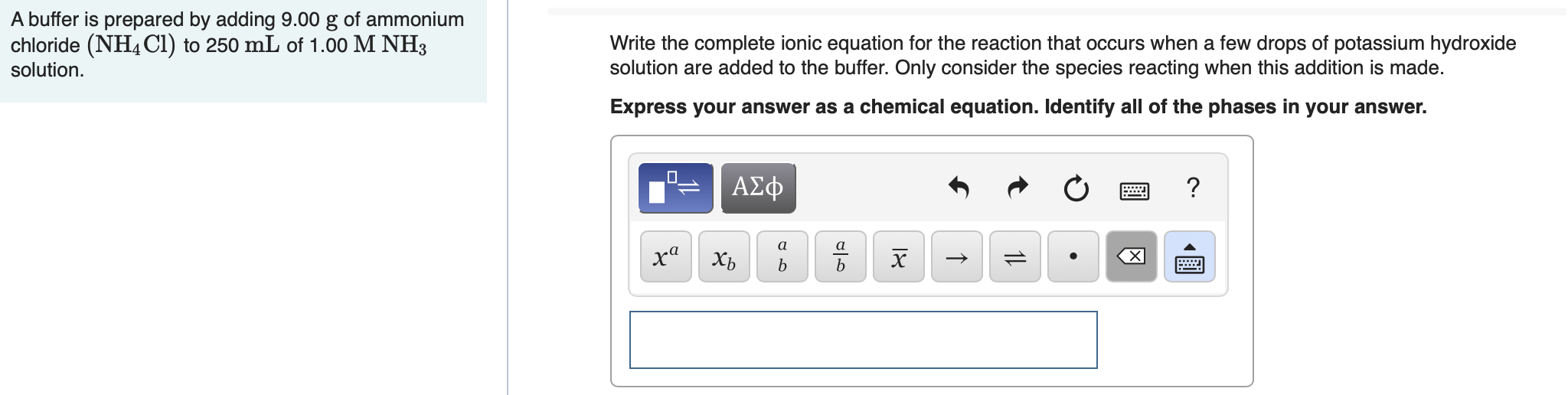
Transcribed Image Text:A buffer is prepared by adding 9.00 g of ammonium
chloride (NH4C1) to 250 mL of 1.00 M NH3
solution.
Write the complete ionic equation for the reaction that occurs when a few drops of potassium hydroxide
solution are added to the buffer. Only consider the species reacting when this addition is made.
Express your answer as a chemical equation. Identify all of the phases in your answer.
ΑΣφ
a
a
ха
х
Хь
1L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

