A buffer solution is 0.40 M in acetic acid and 0.38 M sodium acetate (Ka= 1.75X10). Calculate the change in pH upon adding 30.0 mL of 0.1 M HCI to 200 mL of this solution. 1. O0.06 2. -0.06 3. 45 4. -0.88
A buffer solution is 0.40 M in acetic acid and 0.38 M sodium acetate (Ka= 1.75X10). Calculate the change in pH upon adding 30.0 mL of 0.1 M HCI to 200 mL of this solution. 1. O0.06 2. -0.06 3. 45 4. -0.88
Chapter6: Oral Medication Labels And Dosage Calculation
Section: Chapter Questions
Problem 9.3P
Related questions
Question
I need the answer as soon as possible
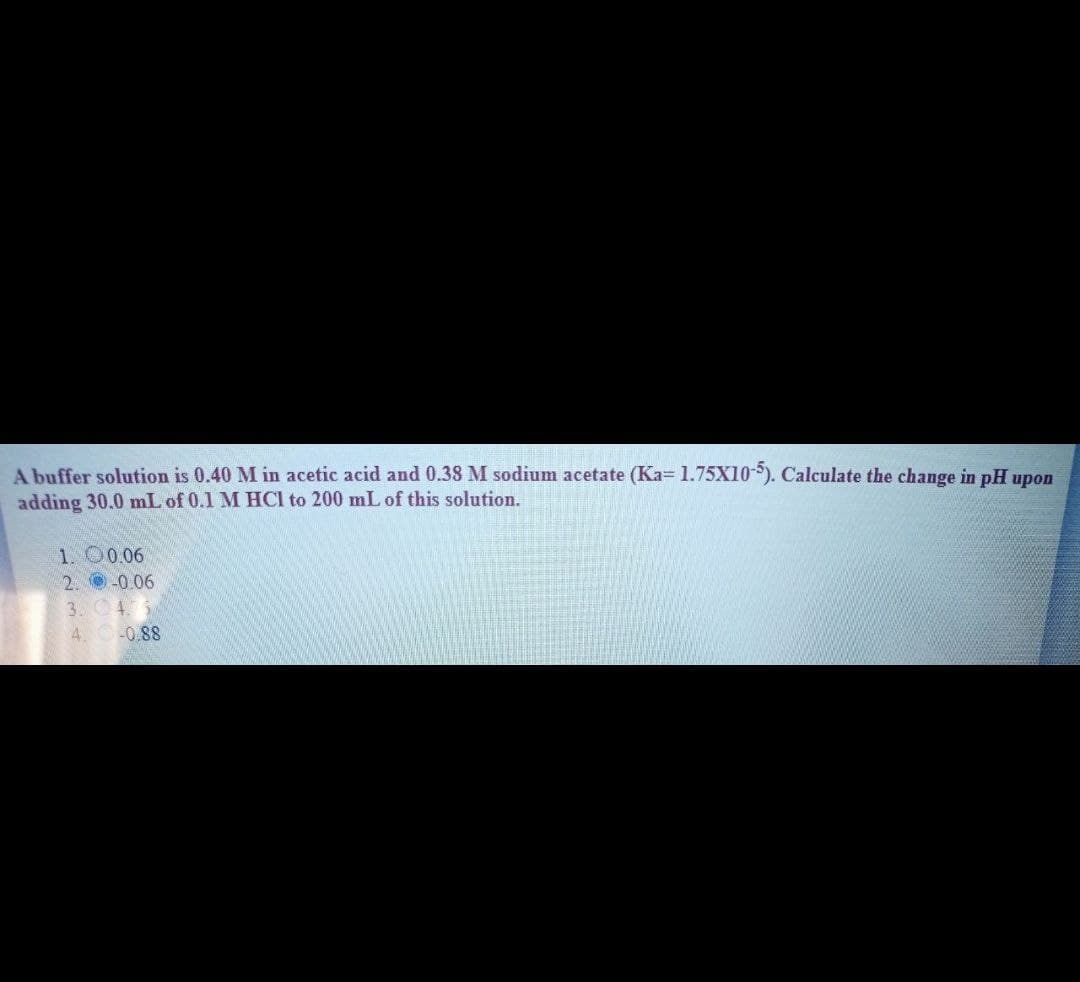
Transcribed Image Text:A buffer solution is 0.40 M in acetic acid and 0.38 M sodium acetate (Ka= 1.75X10*). Calculate the change in pH upon
adding 30.0 mL of 0.1 M HCI to 200 mL of this solution.
1. O0.06
2. -0.06
3. 4.
4 0-0.88
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Basic Clinical Lab Competencies for Respiratory C…
Nursing
ISBN:
9781285244662
Author:
White
Publisher:
Cengage



Basic Clinical Lab Competencies for Respiratory C…
Nursing
ISBN:
9781285244662
Author:
White
Publisher:
Cengage

