Chemistry & Chemical Reactivity 10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
1 Basic Concepts Of Chemistry 2 Atoms Molecules And Ions 3 Chemical Reactions 4 Stoichiometry: Quantitative Information About Chemical Reactions 5 Principles Of Chemical Reactivity: Energy And Chemical Reactions 6 The Structure Of Atoms 7 The Structure Of Atoms And Periodic Trends 8 Bonding And Molecular Structure 9 Bonding And Molecular Structure: Orbital Hybridization And Molecular Orbitals 10 Gases And Their Properties 11 Intermolecular Forces And Liquids 12 The Solid State 13 Solutions And Their Behavior 14 Chemical Kinetics: The Rates Of Chemical Reactions 15 Principles Of Chemical Reactivity: Equilibria 16 Principles Of Chemical Reactivity: The Chemistry Of Acids And Bases 17 Principles Of Chemical Reactivity: Other Aspects Of Aqueous Equilibria 18 Principles Of Chemical Reactivity: Entropy And Free Energy 19 Principles Of Chemical Reactivity: Electron Transfer Reactions 20 Environmental Chemistry-earth's Environment, Energy, And Sustainability 21 The Chemistry Of The Main Group Elements 22 The Chemistry Of The Transistion Elements 23 Carbon: Not Just Another Element 24 Biochemistry 25 Nuclear Chemistry Chapter17: Principles Of Chemical Reactivity: Other Aspects Of Aqueous Equilibria
17.1 The Common Ion Effect 17.2 Controlling Ph: Buffer Solutions 17.3 Acid-base Titrations 17.4 Solubility Of Salts 17.5 Precipitation Reactions 17.6 Equilibria Involving Complex Ions Chapter Questions Section: Chapter Questions
Problem 1PS: Does the pH of the solution increase, decrease or stay the same when you (a) add solid ammonium... Problem 2PS: Does the pH of the solution increase, decrease, or stay the same when you (a) add solid sodium... Problem 3PS: What is the pH of a solution that consists of 0.20 M ammonia, NH3, and 0.20 M ammonium chloride,... Problem 4PS: What is the pH of 0.15 M acetic acid to which 1.56 g of sodium acetate, NaCH3CO2 has been added? Problem 5PS: What is the pH of the solution that results from adding 30.0 mL of 0.015 M KOH to 50.0 mL of 0.015 M... Problem 6PS: What is the pH of the solution that results from adding 25.0 mL of 0.12 M HCl to 25.0 mL of 0.43 M... Problem 7PS: What is the pH of the buffer solution that contains 2.2 g of NH4Cl in 250 mL of 0.12 M NH3? Is the... Problem 8PS: Lactic acid (CH3CHOHCO2H) is found in sour milk, in sauerkraut, and in muscles after activity. (Ka... Problem 9PS: What mass of sodium acetate, NaCH3CO2, must he added to 1.00 L of 0.10 M acetic acid to give a... Problem 10PS: What mass of ammonium chloride, NH4Cl, must be added to exactly 5.00 102 mL of 0.10 M NH3 solution... Problem 11PS: Calculate the pH of a solution that has an acetic acid concentration of 0.050 M and a sodium acetate... Problem 12PS: Calculate the pH of a solution that has an ammonium chloride concentration of 0.050 M and an ammonia... Problem 13PS: What must the ratio of acetic acid to acetate ion be to have a buffer with a pH value of 5.00? Problem 14PS: What must the ratio of H2PO4 to HPO42 be to have a buffer with a pH value of 7.00? Problem 15PS: A buffer is composed of formic acid and its conjugate base, the formate ion. (a) What is the pH of a... Problem 16PS: A buffer solution is composed of 1.360 g of KH2PO4 and 5.677 g of Na2HPO4. (a) What is the pH of the... Problem 17PS: Which of the following combinations would be the best to buffer the pH of a solution at... Problem 18PS: Which of the following combinations would be the best to buffer the pH of a solution at... Problem 19PS: Describe how to prepare a buffer solution from NaH2PO4 and Na2HPO4 to have a pH of 7.5. Problem 20PS: Describe how to prepare a buffer solution from NH3 and NH4Cl to have a pH of 9.5. Problem 21PS: Determine the volume (in mL) of 1.00 M NaOH that must be added to 250 mL of 0.50 M CH3CO2H to... Problem 22PS: Determine the volume (in mL) of 1.00 M HC1 that must be added to 750 mL of 0.50 M HPO42 to produce a... Problem 23PS: A buffer solution was prepared by adding 4.95 g of sodium acetate, NaCH3CO2, to 2.50 102 mL of... Problem 24PS: You dissolve 0.425 g of NaOH in 2.00 L of a buffer solution that has [H2PO4| = [HPO42] = 0.132 M.... Problem 25PS: A buffer solution is prepared by adding 0.125 mol of ammonium chloride to 5.00 102 mL of 0.500 M... Problem 26PS: What is the pH change when 20.0 mL of 0.100 M NaOH is added to 80.0 mL of a buffer solution... Problem 27PS: Phenol, C6H5OH, is a weak organic acid. Suppose 0.515 g of the compound is dissolved in enough water... Problem 28PS: Assume you dissolve 0.235 g of the weak acid benzoic acid, C6H5CO2H, in enough water to make 1.00 ... Problem 29PS: You require 36.78 mL of 0.0105 M HCl to reach the equivalence point in the titration of 25.0 mL of... Problem 30PS: A titration of 25.0 mL of a solution of the weak base aniline, C6H5NH2, requires 25.67 mL of 0.175 M... Problem 31PS: Without doing detailed calculations, sketch the curve for the titration of 30.0 mL of 0.10 M NaOH... Problem 32PS: Without doing detailed calculations, sketch the curve for the titration of 50 mL of 0.050 M... Problem 33PS: You titrate 25.0 mL of 0.10 M NH3 with 0.10 M HCl. (a) What is the pH of the NH3 solution before the... Problem 35PS: Using Figure 17.11, suggest an indicator to use in each of the following titrations: (a) The weak... Problem 36PS: Using Figure 17.11, suggest an indicator to use in each of the following titrations. (a) NaHCO3 is... Problem 37PS: Name two insoluble salts of each of the following ions. (a) Cl (b) Zn2+ (c) Fe2+ Problem 38PS Problem 39PS: Using the solubility guidelines (Figure 3.10), predict whether each of the following is insoluble or... Problem 40PS: Predict whether each of the fallowing is insoluble or soluble in water. (a) Pb(NO3)2 (b) Fe(OH)3 (c)... Problem 41PS: For each of the following insoluble salts, (1) write a balanced equation showing the equilibrium... Problem 42PS Problem 43PS: When 1.55 g of solid thallium(I) bromide is added to 1.00 L of water, the salt dissolves to a small... Problem 44PS: At 20 C, a saturated aqueous solution of silver acetate, AgCH3CO2, contains 1.0 g of the silver... Problem 45PS: When 250 mg of SrF2, strontium fluoride, is added to 1.00 L of water, the salt dissolves to a very... Problem 46PS: Calcium hydroxide, Ca(OH)2, dissolves in water to the extent of 1.78 g per liter. What is the value... Problem 47PS: You add 0.979 g of Pb(OH)2 to 1.00 L of pure water at 25 C. The pH is 9.15. Estimate the value of... Problem 48PS: You place 1.234 g of solid Ca(OH)2 in 1.00 L of pure water at 25 C. The pH of the solution is found... Problem 49PS: Estimate the solubility of silver iodide in pure water at 25 C (a) in moles per liter and (b) in... Problem 50PS: What is the molar concentration of Au+(aq) in a saturated solution of AuCl in pure water at 25 C?... Problem 51PS Problem 52PS: Estimate the solubility of lead(II) bromide (a) in moles per liter and (b) in grams per liter of... Problem 53PS: The Ksp value for radium sulfate, RaSO4, is 4.2 1011. If 25 mg of radium sulfate is placed in 1.00 ... Problem 54PS: If 55 mg of lead(II) sulfate is placed in 250 mL of pure water, does all of it dissolve? If not, how... Problem 55PS Problem 56PS Problem 57PS: Calculate the molar solubility of silver thiocyanate, AgSCN, in pure water and in water containing... Problem 58PS: Calculate the solubility of silver bromide, AgBr, in moles per liter, in pure water. Compare this... Problem 59PS: Compare the solubility, in milligrams per milliliter, of silver iodide, AgI, (a) in pure water and... Problem 60PS: What is the solubility, in milligrams per milliliter, of BaF2, (a) in pure water and (b) in water... Problem 61PS: Calculate the solubility, in moles per liter, of iron(II) hydroxide, Fe(OH)2, in a solution buffered... Problem 62PS: Calculate the solubility, in moles per liter, of calcium hydroxide, Ca(OH)2, in a solution buffered... Problem 63PS: Which insoluble compound in each pair should be more soluble in nitric acid than in pure water? (a)... Problem 64PS: Which compound in each pair is more soluble in water than is predicted by a calculation from Ksp?... Problem 65PS: You have a solution that has a lead(II) ion concentration of 0.0012 M. If enough soluble... Problem 66PS: Sodium carbonate is added to a solution in which the concentration of Ni2+ ion is 0.0024 M. Will... Problem 67PS: If the concentration of Zn2+ in 10.0 mL of water is 1.63 104 M, will zinc hydroxide, Zn(OH)2,... Problem 68PS: You have 95 mL of a solution that has a lead(II) concentration of 0.0012 M. Will PbCl2 precipitate... Problem 69PS Problem 70PS: Will a precipitate of Mg(OH)2 form when 25.0 mL of 0.010 M NaOH is combined with 75.0 mL of a 0.10 M... Problem 71PS: Zinc hydroxide is amphoteric (Section 16.10). Use equilibrium constants to show that, given... Problem 72PS: Solid silver iodide, AgI, can be dissolved by adding aqueous sodium cyanide. Calculate Knet the... Problem 73PS: What amount of ammonia (moles) must be added to dissolve 0.050 mol of AgCl suspended in 1.0 L of... Problem 74PS: Can you dissolve 15.0 mg of AuCl in 100.0 mL of water if you add 15.0 mL of 6.00 M NaCN? Problem 75PS: What is the solubility of AgCl (a) in pure water and (b) in 1.0 M NH3? Problem 76PS Problem 77GQ Problem 78GQ Problem 79GQ Problem 80GQ: Calculate the hydronium ion concentration and the pH of the solution that results when 20.0 mL of... Problem 81GQ: Calculate the hydronium ion concentration and the pH of the solution that results when 50.0 mL of... Problem 82GQ: For each of the following cases, decide whether the pH is less than 7, equal to 7, or greater than... Problem 83GQ Problem 84GQ: A sample of hard water contains about 2.0 103 M Ca2+. A soluble fluoride-containing salt such as... Problem 85GQ: What is the pH of a buffer solution prepared from 5.15 g of NH4NO3 and 0.10 L of 0.15 M NH3? What is... Problem 86GQ Problem 87GQ: Describe the effect on the pH of the following actions or explain why there is not an effect: (a)... Problem 88GQ: What volume of 0.120 M NaOH must be added to 100. mL of 0.100 M NaHC2O4 to reach a pH of 4.70? Problem 89GQ: A buffer solution is prepared by dissolving 1.50 g each of benzoic acid, C6H5CO2H, and sodium... Problem 90GQ: What volume of 0.200 M HCl must be added to 500.0 mL of 0.250 M NH3 to have a buffer with a pH of... Problem 91GQ: What is the equilibrium constant for the following reaction? AgCl(s)+I(aq)AgI(s)+Cl(aq) Does the... Problem 92GQ: Calculate the equilibrium constant for the following reaction. Zn(OH)2(s)+2CN(aq)Zn(CN)2(s)+2OH(aq)... Problem 93GQ Problem 94GQ: The solubility product constant for calcium oxalate is estimated to be 4 109. What is its... Problem 95GQ: In principle, the ions Ba2+ and Ca2+ can be separated by the difference in solubility of their... Problem 96GQ: A solution contains 0.10 M iodide ion, I, and 0.10 M carbonate ion, CO32. (a) If solid Pb(NO3)2 is... Problem 97GQ: A solution contains Ca2+ and Pb2+ ions, both at a concentration of 0.010 M. You wish to separate the... Problem 98GQ Problem 99GQ Problem 100GQ Problem 101IL: Each pair of ions below is found together in aqueous solution. Using the table of solubility product... Problem 102IL: Each pair of ions below is found together in aqueous solution. Using the table of solubility product... Problem 103IL: The cations Ba2+ and Sr2+ can be precipitated as very insoluble sulfates. (a) If you add sodium... Problem 104IL: You will often work with salts of Fe3+, Pb2+, and Al3+ in the laboratory. (All are found in nature,... Problem 105IL: Aniline hydrochloride, (C6H5NH3)Cl, is a weak acid. (Its conjugate base is the weak base aniline,... Problem 106IL: The weak base ethanolamine. HOCH2CH2NH2, can be titrated with HCl.... Problem 107IL: For the titration of 50.0 mL of 0.150 M ethylamine. C2H5NH2, with 0.100 M HCl, find the pH at each... Problem 108IL: A buffer solution with it pH of 12.00 consists of Na3PO4 and Na2HPO4. The volume of solution is... Problem 109IL: To have a buffer with a pH of 2.50, what volume of 0.150 M NaOH must be added to 100. mL of 0.230 M... Problem 110IL: What mass of Na3PO4 must be added to 80.0 mL of 0.200 M HCl to obtain a buffer with a pH of 7.75? Problem 111IL: You have a solution that contains AgNO3, Pb(NO3)2, and Cu(NO3)2. Devise a separation method that... Problem 112IL Problem 113SCQ: Suggest a method for separating a precipitate consisting of a mixture of solid CuS and solid... Problem 114SCQ Problem 115SCQ Problem 116SCQ: Two acids, each approximately 0.01 M in concentration, are titrated separately with a strong base.... Problem 117SCQ: Composition diagrams, commonly known as alpha plots, are often used to visualize the species in a... Problem 118SCQ: The composition diagram, or alpha plot, for the important acid-base system of carbonic acid, H2CO3,... Problem 119SCQ: The chemical name for aspirin is acetylsalicylic acid. It is believed that the analgesic and other... Problem 120SCQ Problem 89GQ: A buffer solution is prepared by dissolving 1.50 g each of benzoic acid, C6H5CO2H, and sodium...
Related questions
Concept explainers
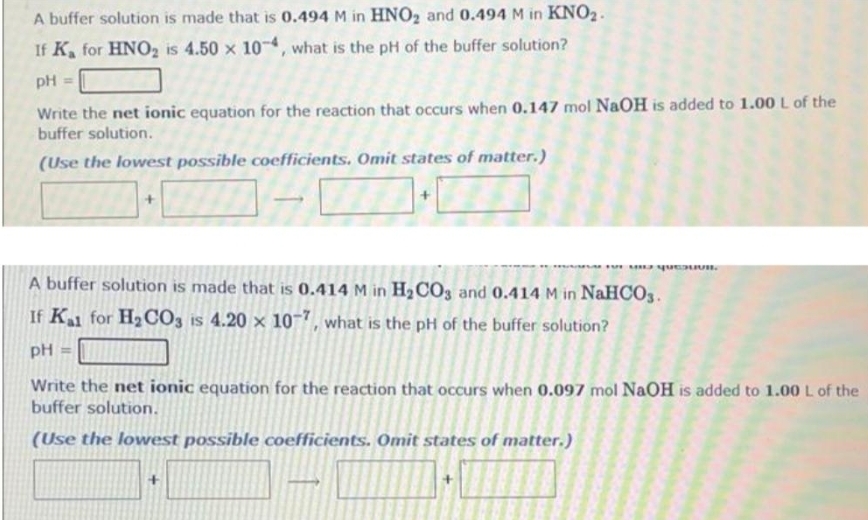
please do all parts. on the net ionic equation, include states of matter.
Transcribed Image Text: A buffer solution is made that is 0.494 M in HNO₂ and 0.494 M in KNO₂.
If K for HNO₂ is 4.50 x 10-4, what is the pH of the buffer solution?
pH =
Write the net ionic equation for the reaction that occurs when 0.147 mol NaOH is added to 1.00 L of the
buffer solution.
(Use the lowest possible coefficients. Omit states of matter.)
FUTEUIU QUEUVR
A buffer solution is made that is 0.414 M in H₂CO3 and 0.414 M in NaHCO3.
If Kal for H₂CO3 is 4.20 x 10-7, what is the pH of the buffer solution?
pH =
Write the net ionic equation for the reaction that occurs when 0.097 mol NaOH is added to 1.00 L of the
buffer solution.
(Use the lowest possible coefficients. Omit states of matter.)
Definition Definition Substance that constitutes everything in the universe. Matter consists of atoms, which are composed of electrons, protons, and neutrons. Different atoms combine together to give rise to molecules that act as a foundation for all kinds of substances. There are five states of matter based on their energies of attraction: solid, liquid, gases, plasma, and BEC (Bose-Einstein condensates).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps